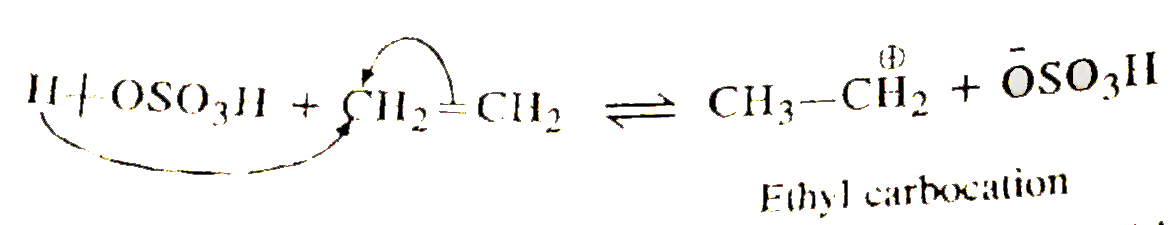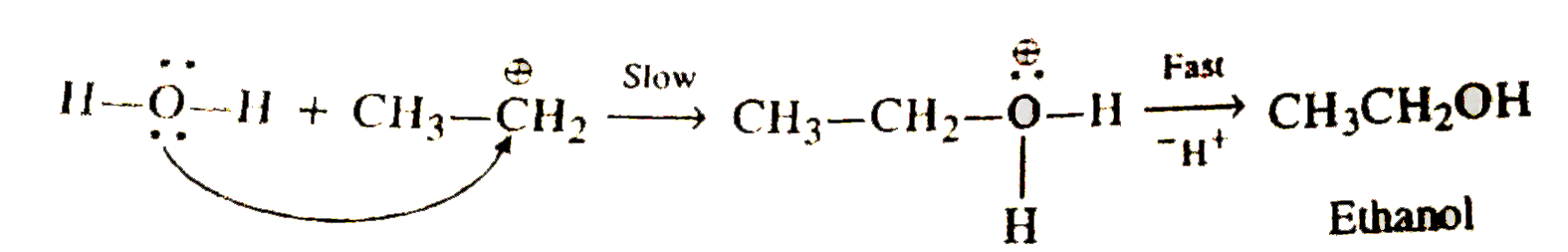Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the mechanism of hydration of ethene to yield ethanol.
Text Solution
|
- Write the mechanism of hydration of ethene to yield ethanol.
Text Solution
|
- Write the mechanism of acid dehydration of ethanol to yield ethene.
Text Solution
|
- एथीन के जलयोजन से ऐथेनॉल प्राप्त करने की क्रिया-विधि लिखिए।
Text Solution
|
- Write the mechanism of hydration of ethane to yield ethanol.
Text Solution
|
- एथीन के जलयोजन से एथेनॉल प्राप्त करने की क्रियाविधि लिखिये-
Text Solution
|
- Wriite the mechanism of hydration of ethene to form ethanol.
Text Solution
|
- ऐथीन के जलयोजन से एथेनॉल प्राप्त करने की क्रियाविधि लिखिये।
Text Solution
|
- Write the mechanism of acid dehydration of ethanol to yield ethene
Text Solution
|