Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
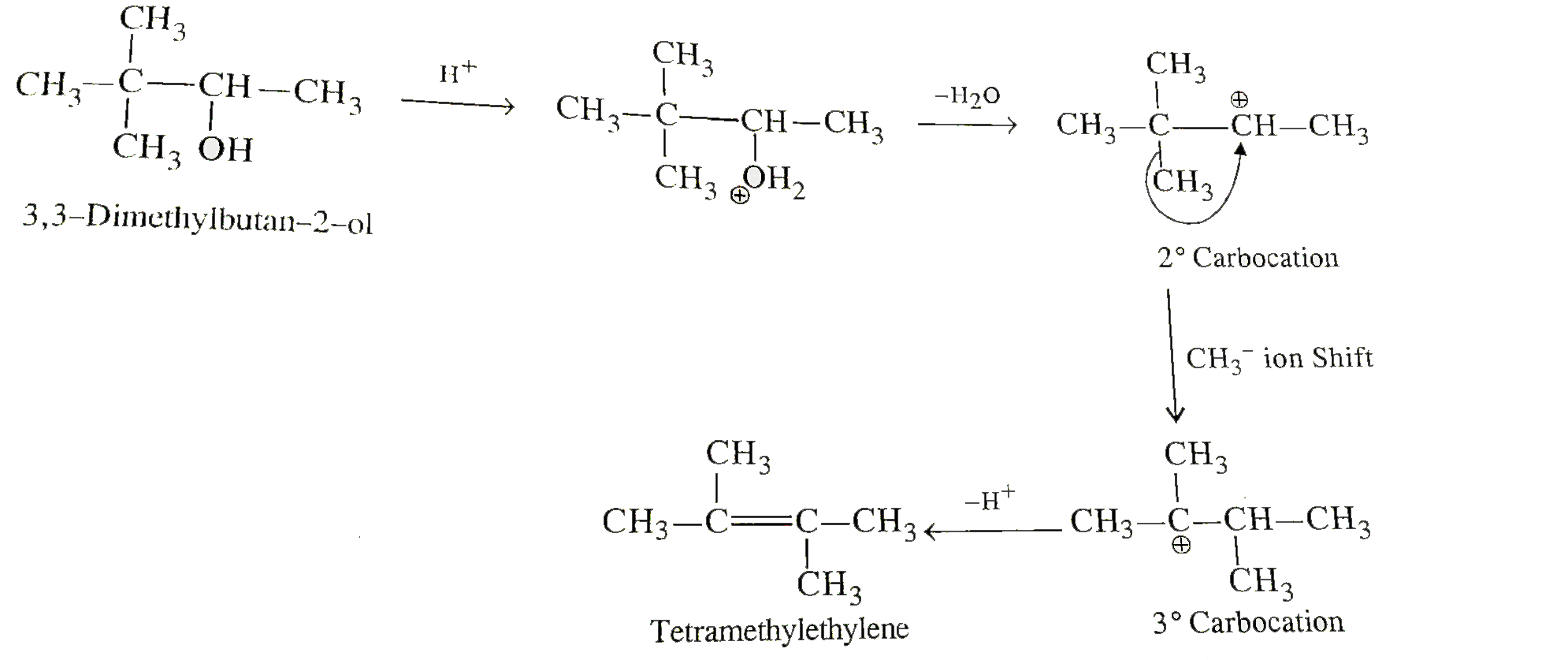
- 3,3-Dimethylbutan-2-of loses a molecule of water in the presence of a ...
Text Solution
|
- The major product formed when 3,3-dimethylbutan-2-ol is heated with co...
Text Solution
|
- Phenol is heated with concentrated sulphuric acid at 110^(@)C . The ma...
Text Solution
|
- The major product formed when 3,3-dimethylbutan-2-ol is heated with co...
Text Solution
|
- 3,3-dimethylbutan-2-ol losses a molecule of water in the presence of c...
Text Solution
|
- The main product produced in the dehydrohalogenation of 2-bromo-3,3-di...
Text Solution
|
- The major product when 3,3-dimethyl-butan-2-ol is heated with concentr...
Text Solution
|
- Explain why the addition of HI to 3,3-dimethylbut-1-ene gives 2-iodo-2...
Text Solution
|
- सान्द्र सल्फ्यूरिक अम्ल की उपस्थिति में 3,3-डाइमेथिल ब्यूटेन-2-ऑल का ए...
Text Solution
|