Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
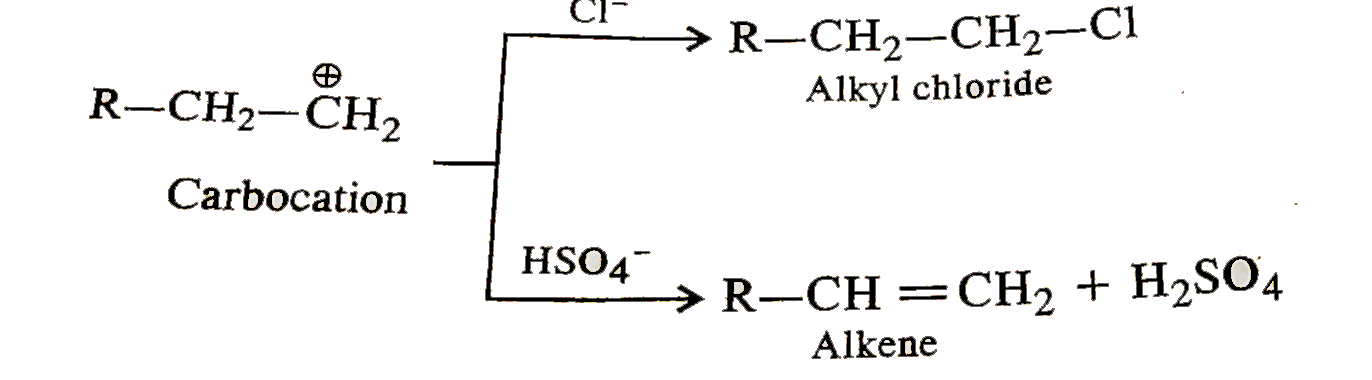
- During dehydration of alcohols to alkenes by heating with conc. H(2) S...
Text Solution
|
- During dehydration of alcohols to alkenes by heating with conc. H(2)SO...
Text Solution
|
- During the dehydration of alcohols to alkenes by heating with conc. H(...
Text Solution
|
- During dehydration of alcohols to alkenes by heating with conc. H(2)SO...
Text Solution
|
- During dehydration of alcholos to alkenes by heating with conc. H(2)SO...
Text Solution
|
- Dehydration of methyl alcohol with conc. H(2)SO(4) yields
Text Solution
|
- During the dehydration of alcohols to alkenes by heating with conc. H(...
Text Solution
|
- During the dehydration of alcohols to alkenes by heating with conc. H(...
Text Solution
|
- During dehydration of alcohols to alkenes by heating with conc. H(2)SO...
Text Solution
|