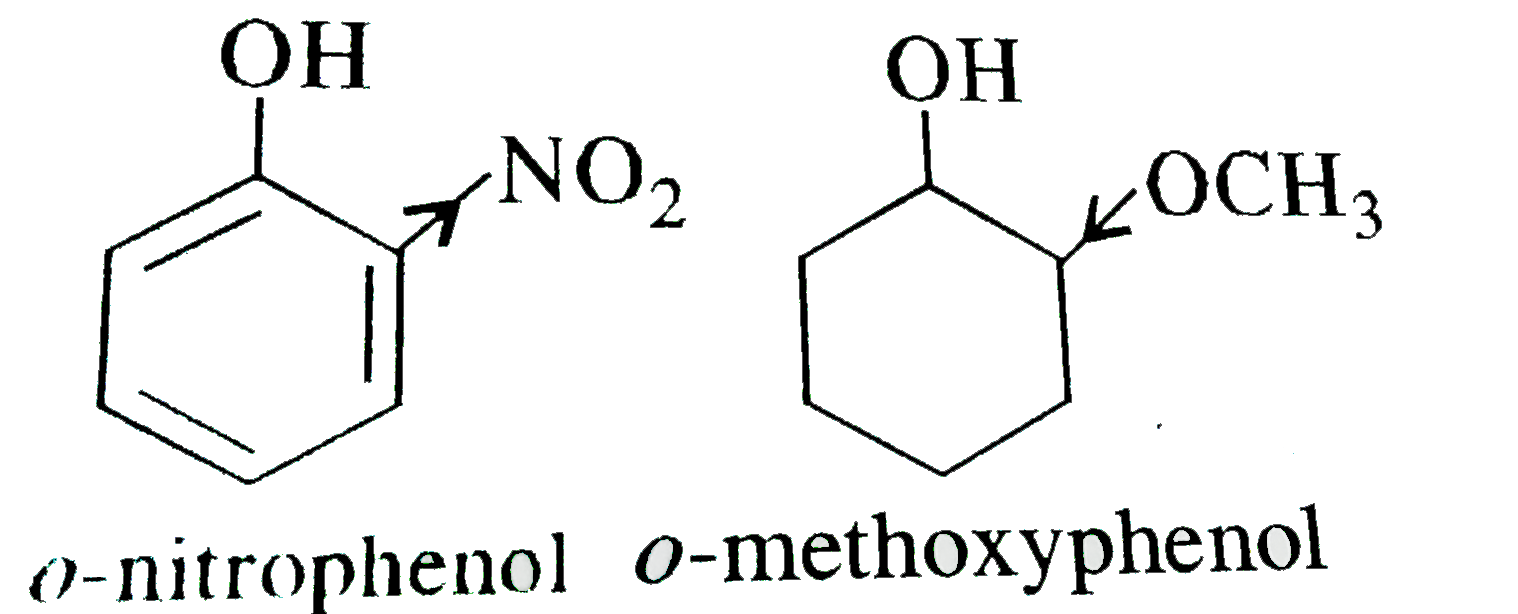
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the order of dehydration of primary, secondary and tertiary a...
Text Solution
|
- Explain the relative ease of dehydration of alcohols as : tertiary gt ...
Text Solution
|
- प्राथमिक, द्वितीयक व तृतीयक ऐल्कोहॉलों की निर्जलन अभिक्रिया का केवल रा...
Text Solution
|
- What is dehydration of alcohols ? Give the chemical reaction showing d...
Text Solution
|
- प्राथमिक , द्वितीयक तथा तृतीयक ऐल्कोहॉल के निर्जलीकरण का क्या क्रम है ...
Text Solution
|
- What is the order of acidic character of primary, secondary and tertia...
Text Solution
|
- What are primary, secondary and tertiary alcohols?
Text Solution
|
- Explain the dehydration of primary, secondary and tertiary alcohols.
Text Solution
|
- प्राथमिक, द्वितीयक तथा तृतीयक ऐल्कोहॉलों की अम्लता का क्रम लिखिए।
Text Solution
|