Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An alkoxide ion is a stronger base than hydroxide ion. Justify.
Text Solution
|
- The hydride ion H^(ɵ) is a stronger base than hydroxide ion. Which of ...
Text Solution
|
- The conjugate base of hydroxide ion is
Text Solution
|
- An alkoxide ion is a stronger base than hydroxide ion. Justify.
Text Solution
|
- The hydride ion H^(-) is a stronger base than its hydroxide ion OH^(-)...
Text Solution
|
- The hydride ion H^(-) is stronger base than its hydroxide ion OH^(-). ...
Text Solution
|
- The hydride ion H^(-) is a stronger base than its hydroxide ion OH^(-)...
Text Solution
|
- The hydride ion H^(-) is stronger base than its hydroxide ion OH^(-). ...
Text Solution
|
- The H^(-) ion is stronger base than hydroxide ion which of the follo...
Text Solution
|
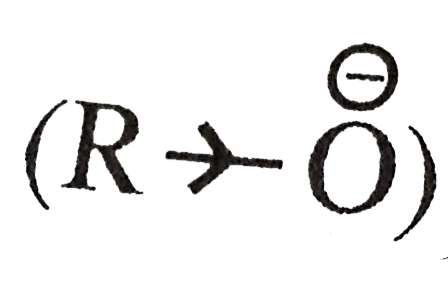 , electron density on the alkoxide ion is moreas compared to hydroxide ion `(OH^(Θ))`. It can therefore, accept a `H^(+)` more easily and is a stronger base than the hydroxide ion.
, electron density on the alkoxide ion is moreas compared to hydroxide ion `(OH^(Θ))`. It can therefore, accept a `H^(+)` more easily and is a stronger base than the hydroxide ion.