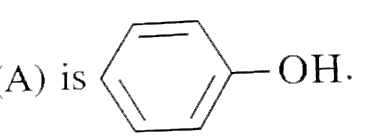
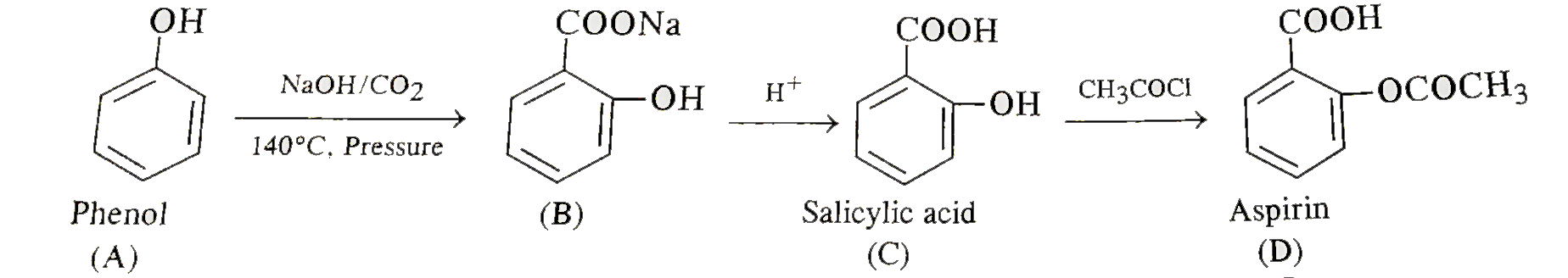Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound (A) having molecular formula C2 HCl3 O reduces Feh...
Text Solution
|
- An organic compound (A) on analysis was found to contain C = 16.271%, ...
Text Solution
|
- An organic compound (A) of molecular formula C(7)H(6)O is not reduced ...
Text Solution
|
- An organic compound A of molecular formula C2H4 on reduction gives ano...
Text Solution
|
- A compound A has a molecular formula C2Cl3OH. It reduces Fehling solut...
Text Solution
|
- An organic compound A of molecular formula CH2O reacts with methyl ma...
Text Solution
|
- An organic compound (A) of molecular formula CH4O on mild oxidation gi...
Text Solution
|
- An organic compound (A) is a calcium salt of acetic acid. (A) on dry d...
Text Solution
|
- An aromatic aldehyde (A) of molecular C7H6O reacts with acidified KMnO...
Text Solution
|