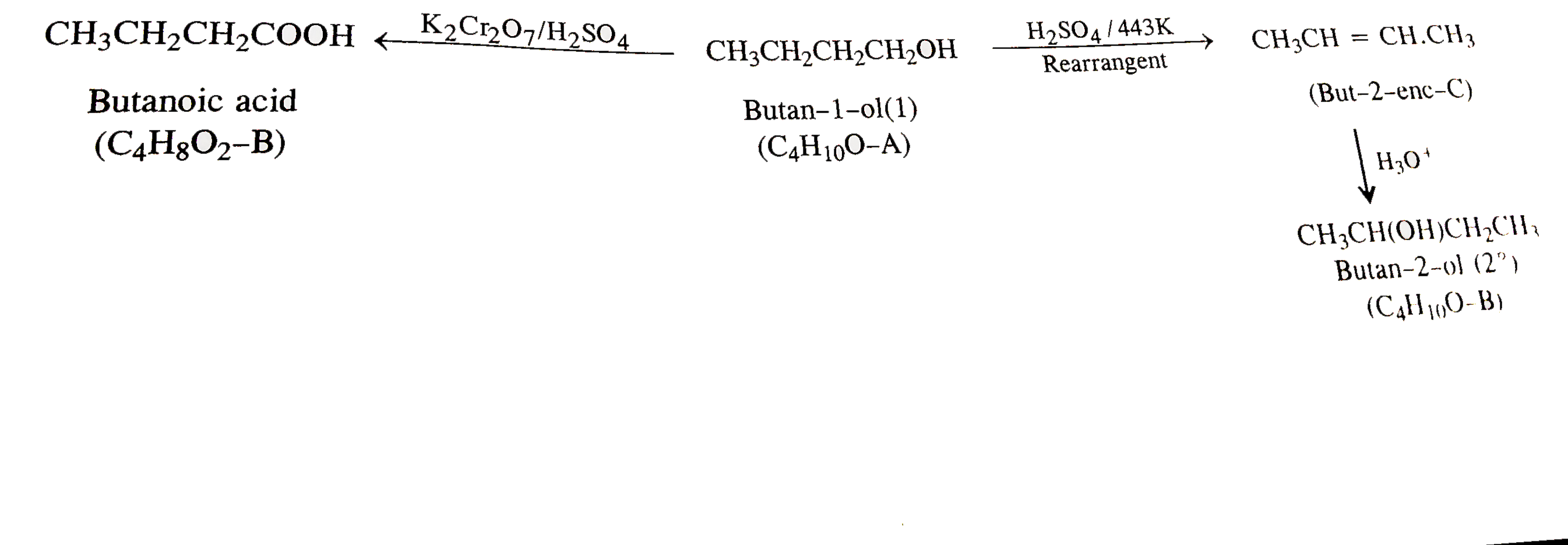Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An alcohol A(C(4)H(10)O) on oxidation with acidified potassium dichrom...
Text Solution
|
- An organic compound A (C(7)H(6)Cl(2)) on treatment with NaOH solution ...
Text Solution
|
- An alcohol [A] with molecules formula (C(4)H(10)O) o oxidation with ac...
Text Solution
|
- A substance C(4)H(10)O yields on oxidation a compound C(4)H(8)O which ...
Text Solution
|
- A substance C(4)H(10)O yields on oxidation a compound C(4)H(8)O which ...
Text Solution
|
- An organic compound [A] C(6)H(10), on reduction first gives [B] C(6)H(...
Text Solution
|
- A substance C(4)H(10)O yields on oxidation a compound C(4)H(8)O which ...
Text Solution
|
- An organic compound (A) of molecular formula C(5)H(8) when treated wit...
Text Solution
|
- A substance C(4)H(10)O yield on oxidation C(4)H(5)O which gives oxime ...
Text Solution
|