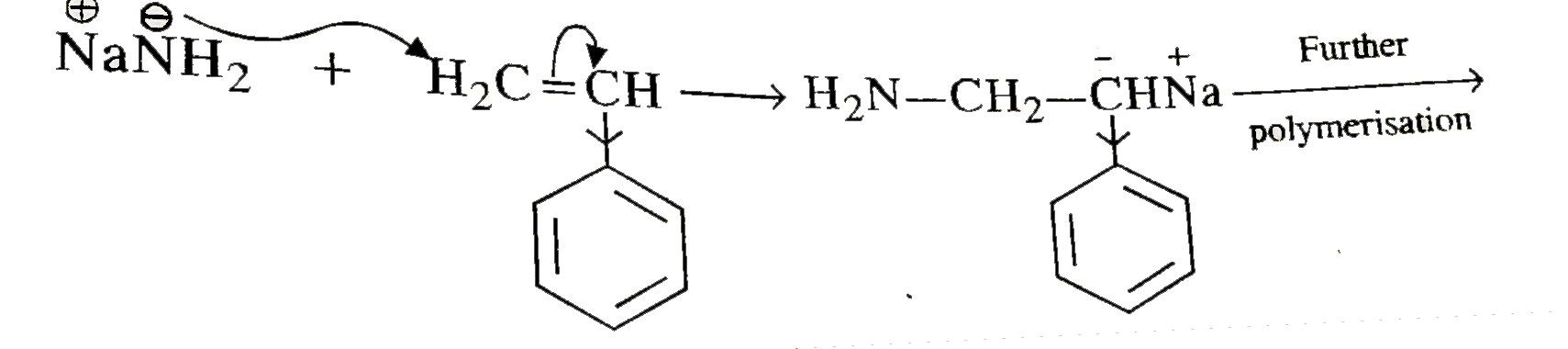Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Why styrene undergo anionic polymerisation easily.
Text Solution
|
- Which of the following polymerises most easily ?
Text Solution
|
- The monomer that undergo radical polymerisation most easily is :
Text Solution
|
- Why does styrene undergo anionic polymerisation easily ?
Text Solution
|
- 1,3-Butadine and styrene on polymerisation give:
Text Solution
|
- स्टाइरीन शीघ्रता से ऋणारयनिक बहुलकन में भाग क्यों लेती है ?
Text Solution
|
- Name any two monomers that talce part in anionic polymerisation. Why i...
Text Solution
|
- During the polymerisation of styrene, the radical formed in first step...
Text Solution
|
- Toluene undergoes nitration easily than Benzene why?
Text Solution
|