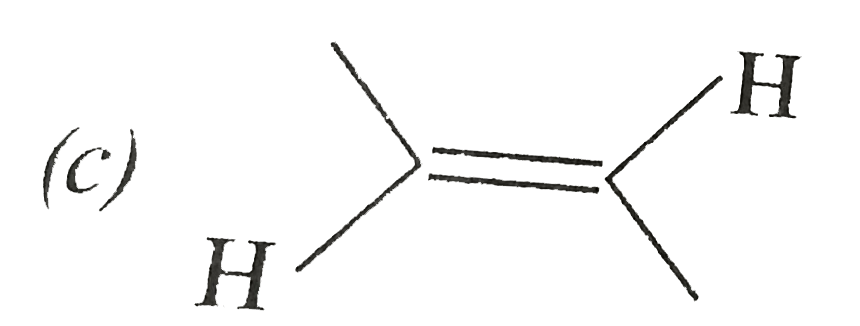
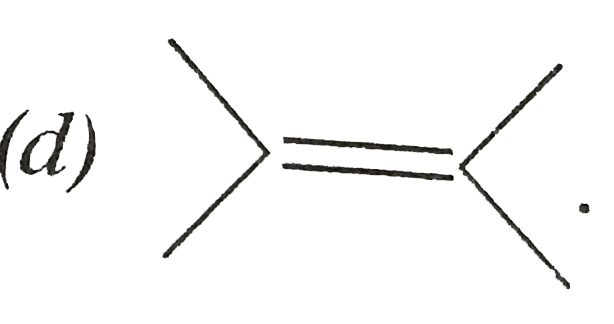
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The monomer of the polymer ~CH(2)-underset(CH(3))underset(|)overset(...
Text Solution
|
- The monomer of the polymer ~CH(2)-underset(CH(3))underset(|)overset(...
Text Solution
|
- Write the IUPAc name of the following compound : (a) CH(3)-CH(2)-ov...
Text Solution
|
- Monomer of given polymer [-underset(underset(CH(3))(|))overset(overset...
Text Solution
|
- Write the structures of monomers of the following polymers (a) [-CH(...
Text Solution
|
- CH(3)-underset(OH)underset(|)overset(CH(3))overset(|)(C)-underset(NH(2...
Text Solution
|
- The IUPAC name of CH(3)-underset(CH(3))underset(|)overset(CH(3))overse...
Text Solution
|
- (--CH(2)-overset(CH(3))overset("| ")underset(CH(3))underset("| ")"C "-...
Text Solution
|
- (-CH(2) - underset(CH(3))underset(||)overset(CH(3))overset(||)(C) - CH...
Text Solution
|