A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The total number of lone-pairs of electrons in melamine is.
Text Solution
|
- The total number of lone-paris of electrons in melamine is
Text Solution
|
- The total number of lone-pairs of electrons in melamine is.
Text Solution
|
- The total number of Ione-pair of electrons in melamine is
Text Solution
|
- Number of pi electrons which are delocalized in melamine is X and numb...
Text Solution
|
- The total number of lone pairs of electrons in melamine is:
Text Solution
|
- Total number of lone pairs of electron in melamine is
Text Solution
|
- The sum of total number of lone - pairs of electrons and sp^(3) hybrid...
Text Solution
|
- मैलेमीन पर उपलब्ध इलेक्ट्रॉनों के एकाकी युग्मों की कुल संख्या है।
Text Solution
|
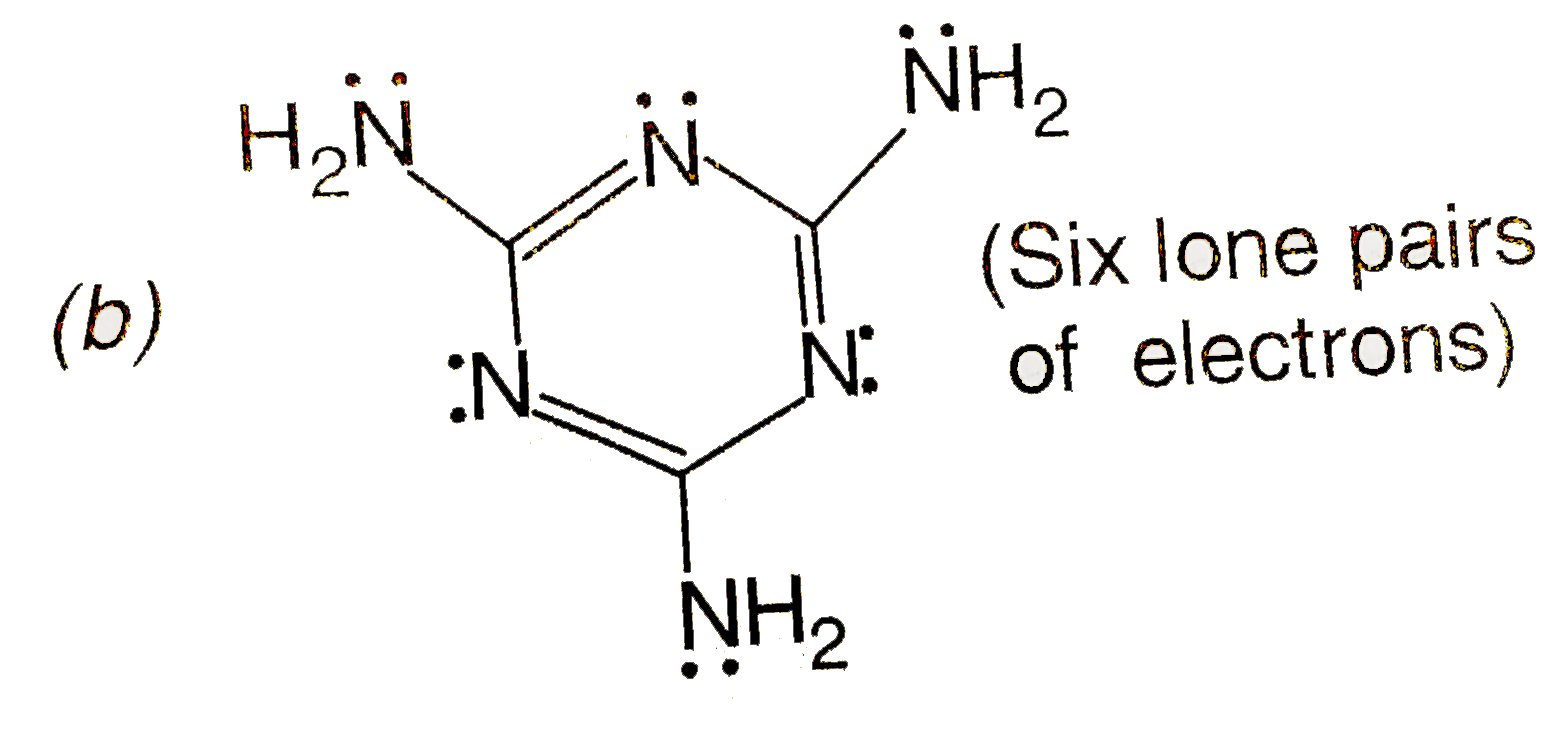 (Six lone pairs of electrons)
(Six lone pairs of electrons)