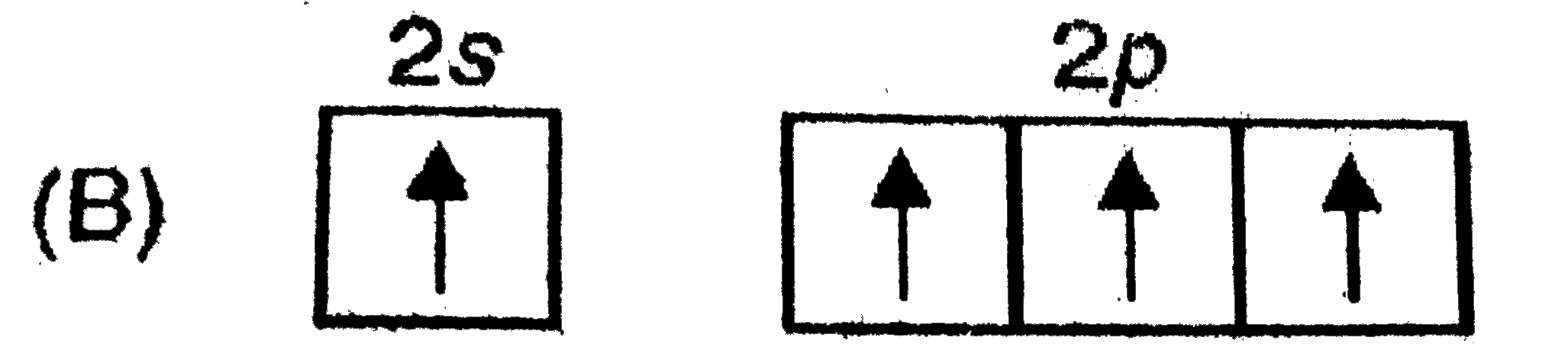
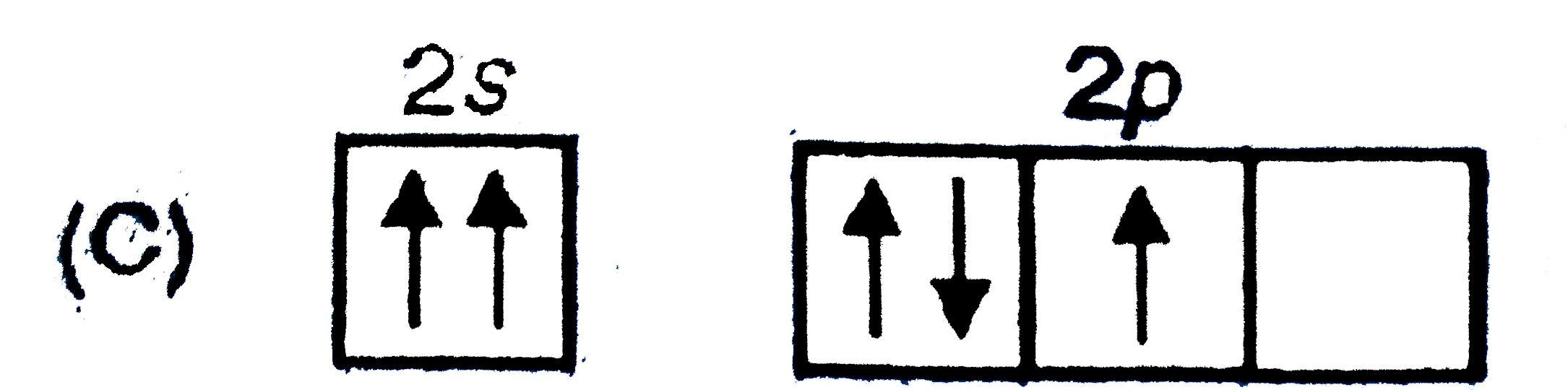
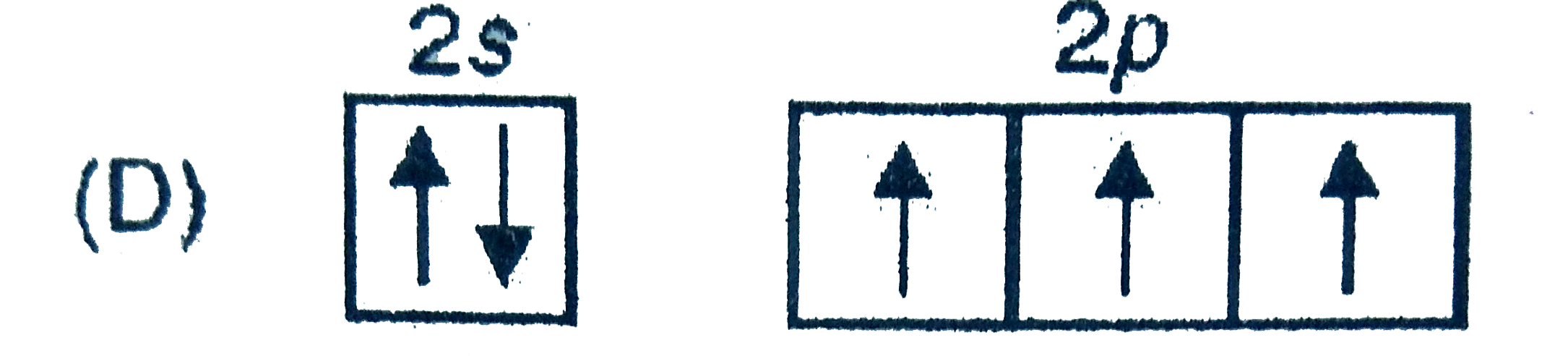
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following electron distribution in ground state, only ...
Text Solution
|
- For which of the following electron distribution in ground state the (...
Text Solution
|
- Which of the following is a violation of the Hund's rule?
Text Solution
|
- Which wriiting the following electron cofiguration of Fe some rule hav...
Text Solution
|
- Hund's rule states that the most stable arrangement of electrons : (fo...
Text Solution
|
- which of the following electrons configuraation violate Hund's rule fo...
Text Solution
|
- In which of the following electron distribution in ground state, only ...
Text Solution
|
- Which of the following is violating hund's rule :-
Text Solution
|
- The eleectronic cofiguration which obeys Hund's rule for the ground s...
Text Solution
|