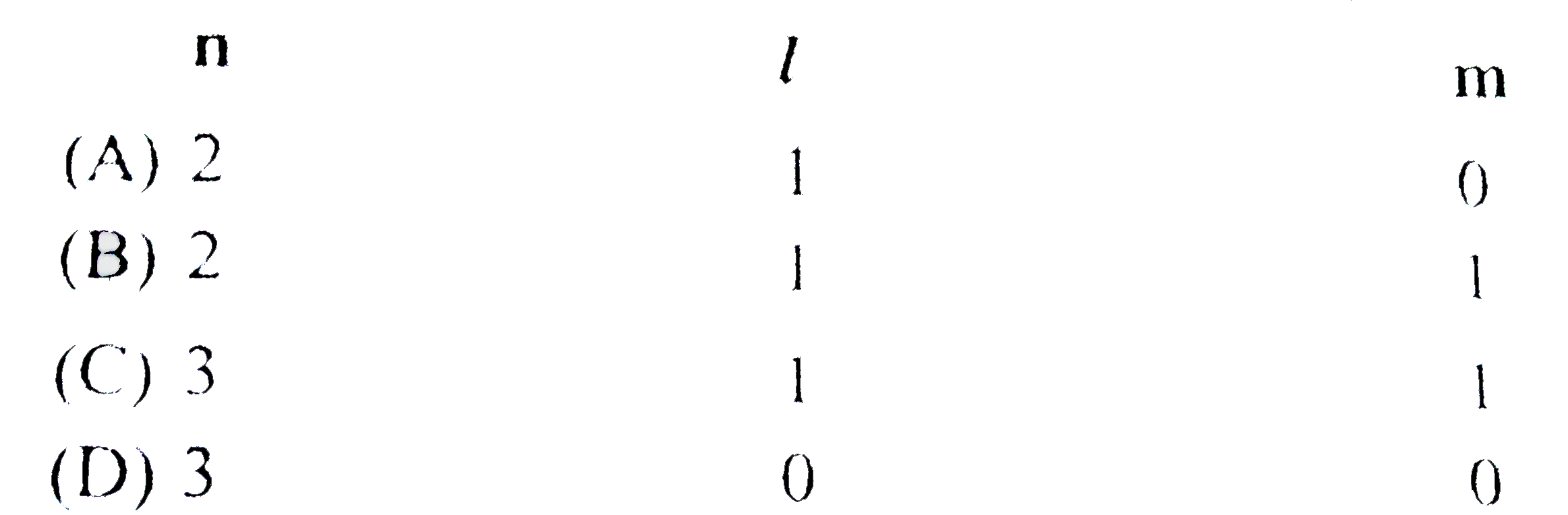
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- The correct set of quantum number for the unpaired electron of a chlor...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- क्लोरीन परमाणु के अयुग्मित इलेक्ट्रॉन की क्वाण्टम संख्याओं का कौन -सा ...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of a chlo...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|