A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The representation of the ground state electronic configuration of He ...
Text Solution
|
- For which of the following electron distribution in ground state the (...
Text Solution
|
- The ground state electronic configuration of platinum is
Text Solution
|
- In which of the following electron distribution in ground state, only ...
Text Solution
|
- The representation of the ground state electronic configuration of He ...
Text Solution
|
- The representation of the ground state electronic configuration of He ...
Text Solution
|
- If the Nitrogen atom had electronic configuration 1s^(7), it would hav...
Text Solution
|
- The following electronic configuration violates
Text Solution
|
- The following electronic configuration violates
Text Solution
|
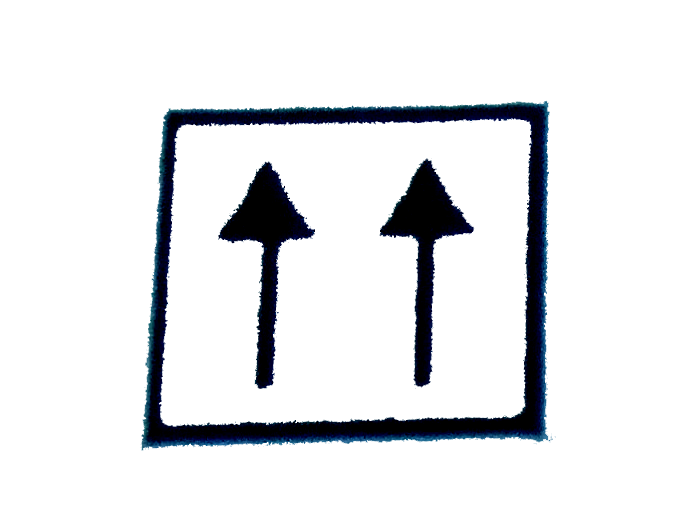 is wrong because it violates
is wrong because it violates