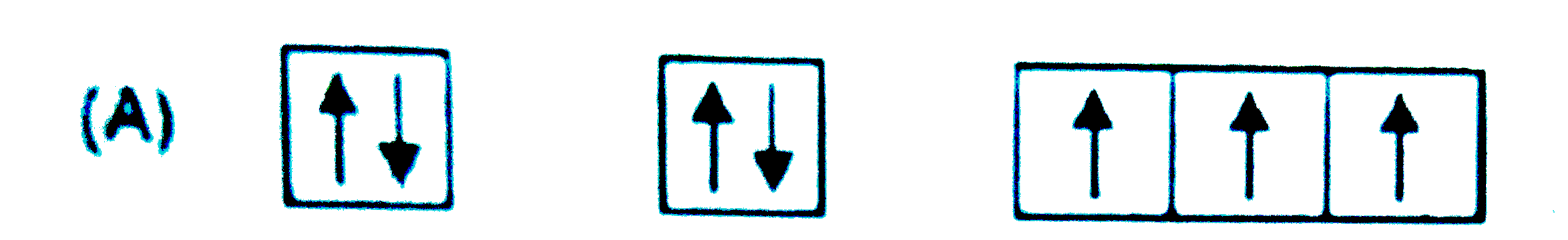
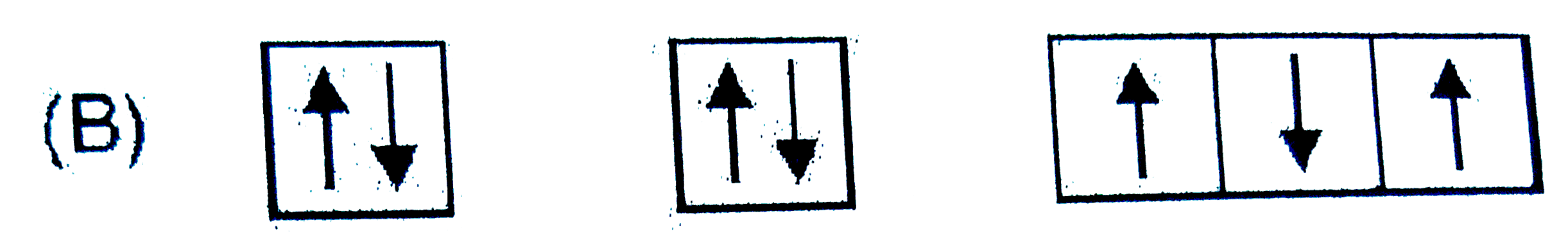
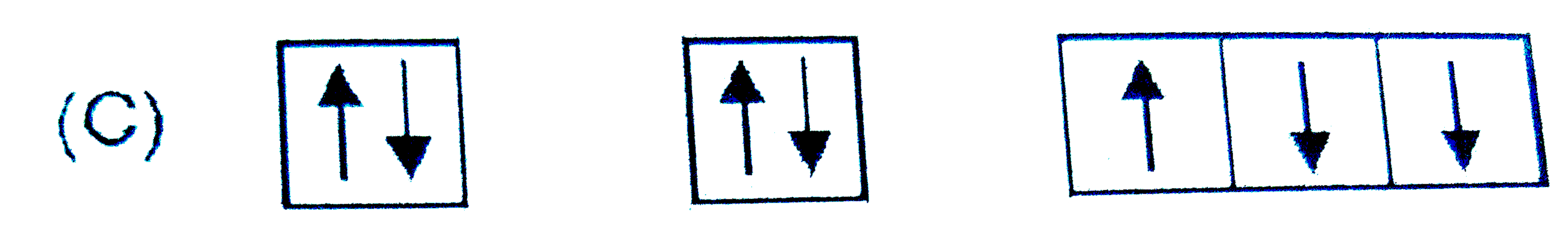
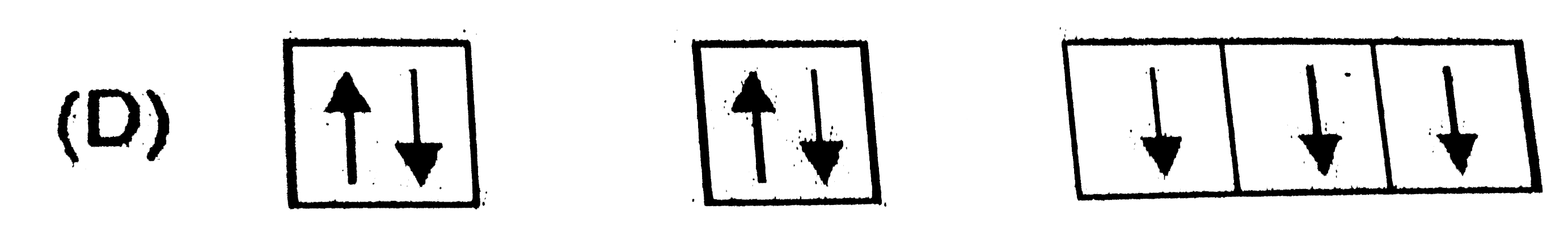
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The gaseous state electronic configuration of nitrogen atom can be rep...
Text Solution
|
- Assertion (A) : The electronic configuration of nitrogen atom is repre...
Text Solution
|
- the ground state valence shell electrons configuration of nitrogen ato...
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- Gaseous state electronic configuration of ntrogen atom can be represen...
Text Solution
|
- नाइट्रोजन परमाणु के मूल अवस्था में इलेक्ट्रॉनिक विन्यास को निम्न प्रका...
Text Solution
|
- The ground state electronic configuration of nitrogen atom can be repr...
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|