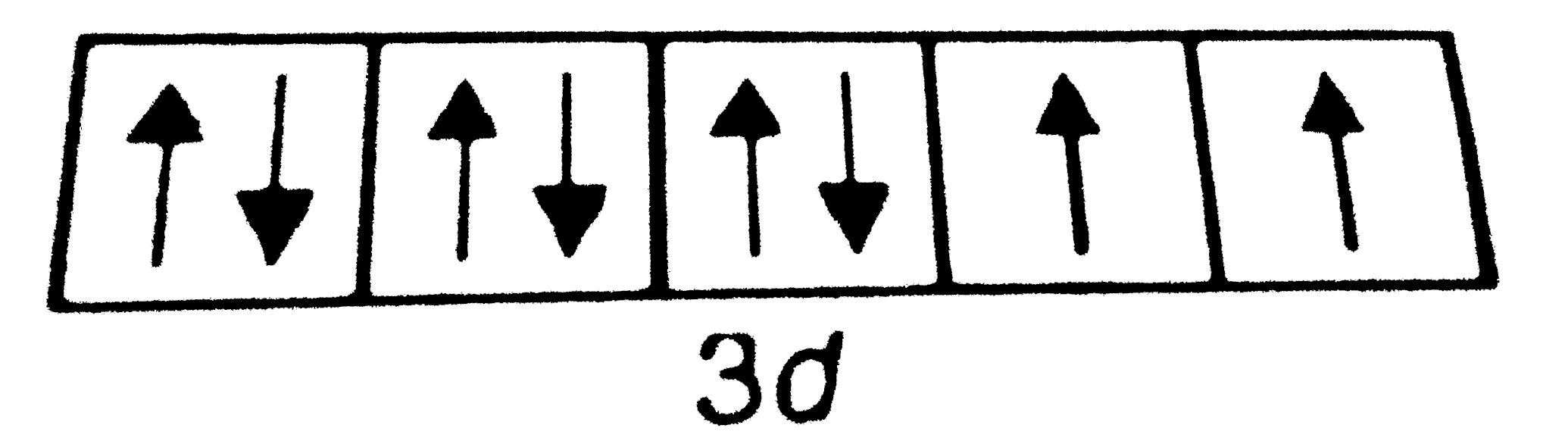
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many unpaired electrons are there in Ni^(2+) ion if the atomic num...
Text Solution
|
- How many unpaired electrons are there in Ni^(2+) ?
Text Solution
|
- The number of unpaired electrons in a nickel atom (ground state) are (...
Text Solution
|
- Write down the electronic configurations of Ni and Ni^(2+) (atomic num...
Text Solution
|
- The number of unpaired electrons in Ni (atomic number=28) are-
Text Solution
|
- How many unpaired electrons are present in Ni^(2+) cation? (At. No. = ...
Text Solution
|
- Ni^(2+) आयन (Z = 28) में अयुग्मित इलेक्ट्रॉनों की संख्या है
Text Solution
|
- How many unpaired electrons are there is Ni^(2+)
Text Solution
|
- " How many unpaired electrons are present in "(Ni^(2))" .cation (atomi...
Text Solution
|