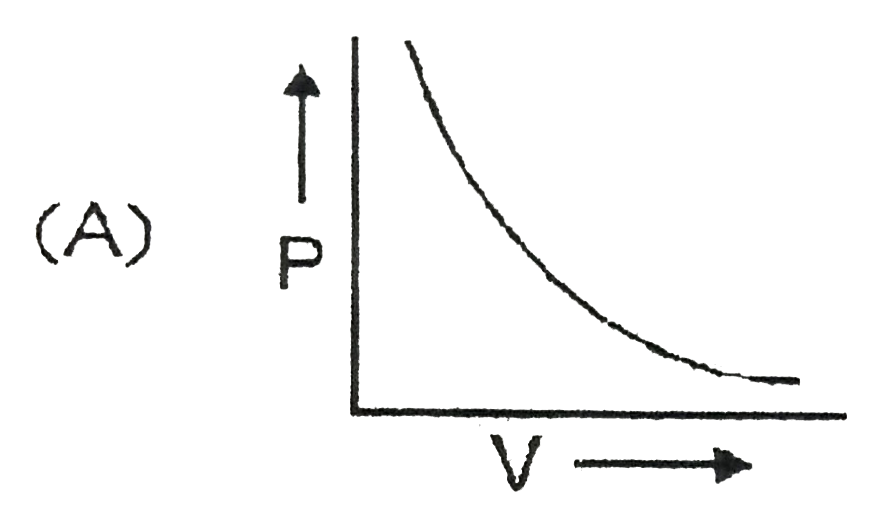
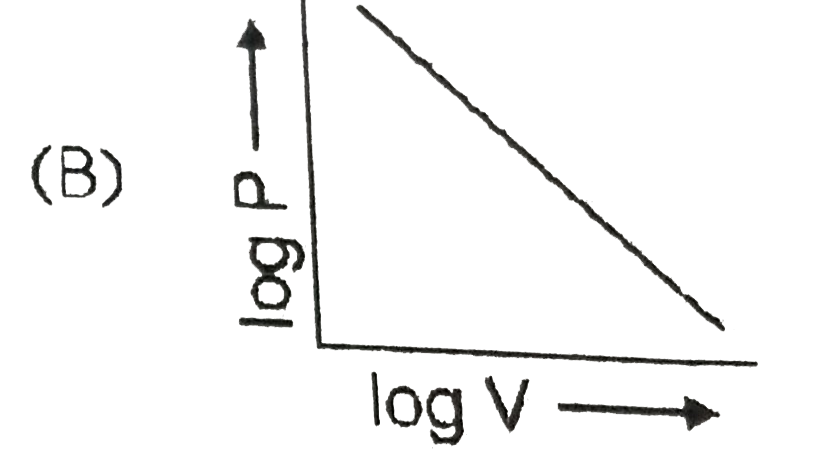
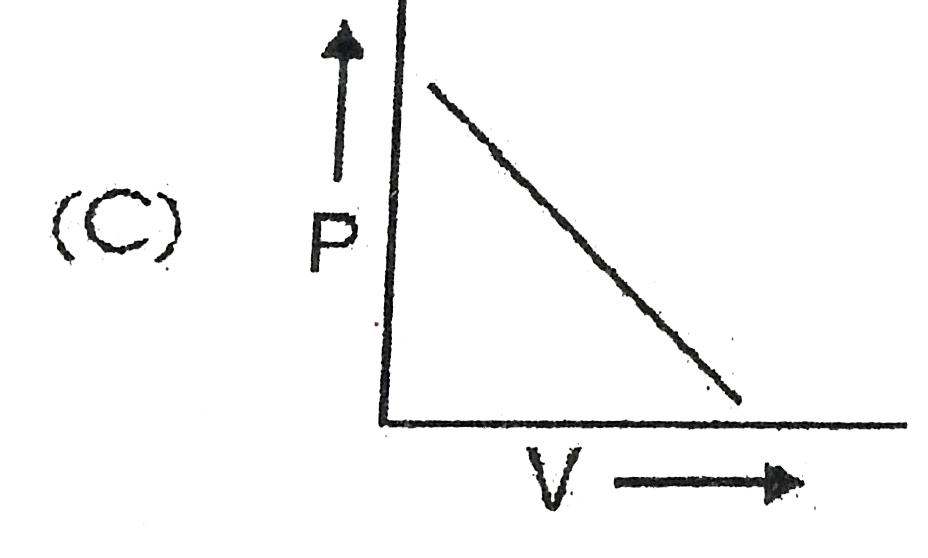
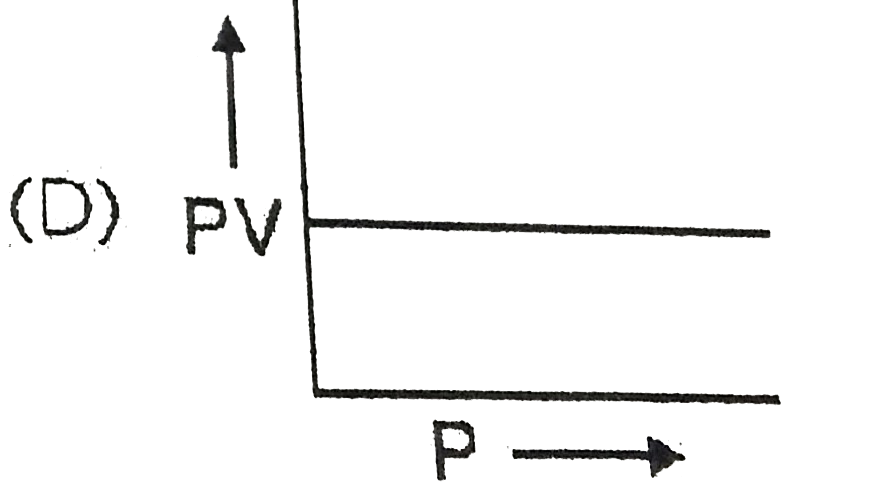
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which curve does not represent Boyl's law ?
Text Solution
|
- Which of the following curves does not represent Boyle's law?
Text Solution
|
- Which of the following curves represents the Henry's law ?
Text Solution
|
- Which of the following curves represents the Henry's law?
Text Solution
|
- Which of the following curves represent the Henry's law
Text Solution
|
- Which curve does not represent Boyl's law ?
Text Solution
|
- Which of the following curve does not represent Gay-Iusacc's law?
Text Solution
|
- Which of the following curves represent(s) Boyle's law?
Text Solution
|
- Which curve does not represent Boyle’s law
Text Solution
|