A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
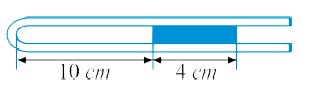
- A 10 cm column of air is trapped by a column of Hg 4 cm long in a capi...
Text Solution
|
- If above tube is held vertical with open end upward then find the leng...
Text Solution
|
- A 10.0 cm column of air is trapped by a column of Hg 4.00 cm long in c...
Text Solution
|
- Air is trapped in a horizontal glass tube by 36 cm mercury column as s...
Text Solution
|
- A 10 cm column of air is trapped by a column of Hg, 8 cm long, in a ca...
Text Solution
|
- In a capillary tube,closed at one end,some air is enclosed by a mercur...
Text Solution
|
- A 10 cm column of air is trapped by a column of mercury, 8 cm long, in...
Text Solution
|
- A 10 cm column of air is trapped by a column of mercury, 8 cm long, in...
Text Solution
|
- A 10 cm column of air is trapped by a column of mercury, 8 cm long, in...
Text Solution
|