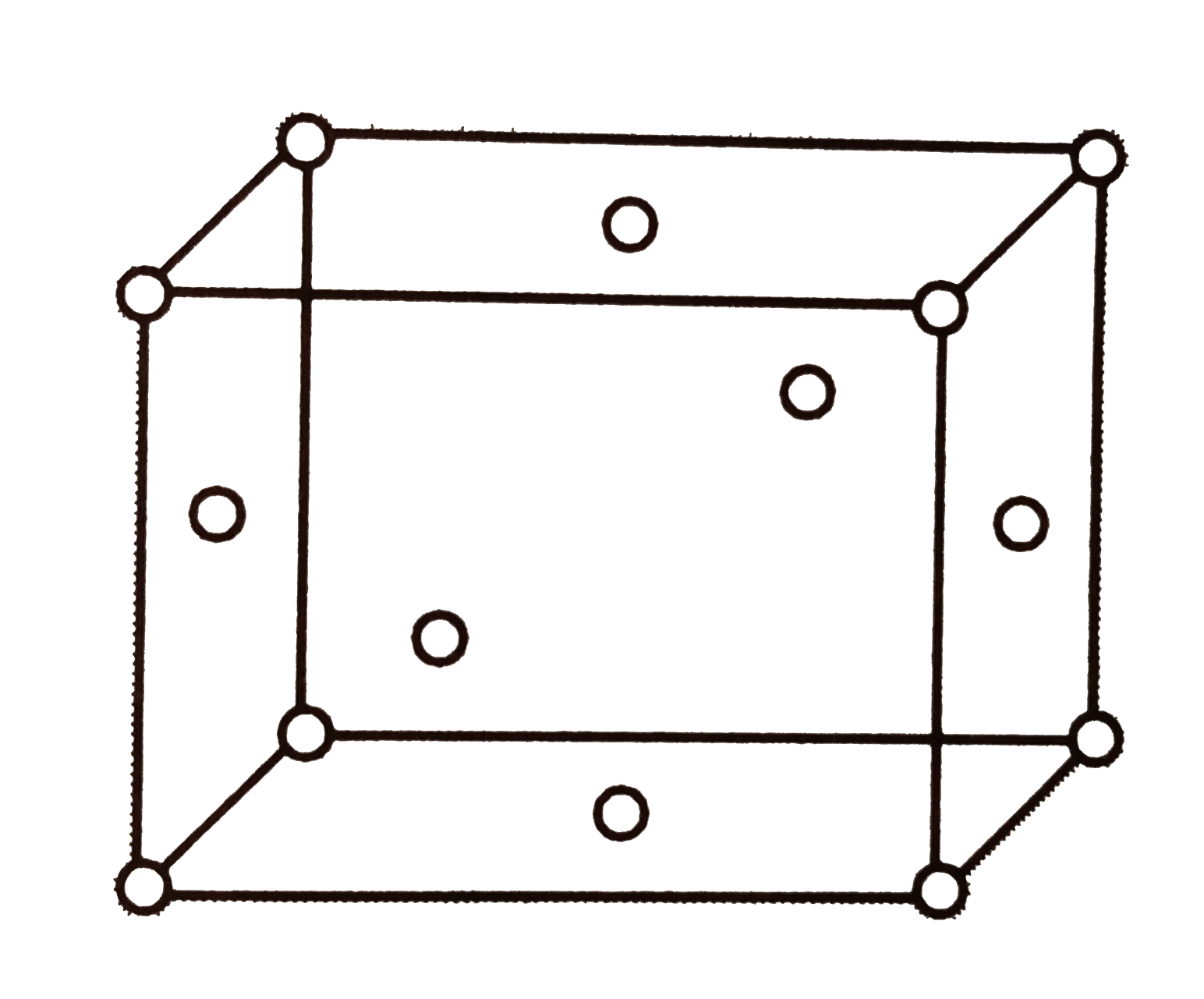
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the structure of solid given below, if the lattice points represen...
Text Solution
|
- For the structure of solid given below, if the lattice points represen...
Text Solution
|
- A solide A^(+)B^(-) has the B^(-) ions arranged as below. If the A^(+)...
Text Solution
|
- If the crystallises in zinc blende structure with I^- ions at lattice ...
Text Solution
|
- If the crystallises in zinc blende structure with I^- ions at lattice ...
Text Solution
|
- A binary solid A^(+) B^(-) has a structure with B^(-) ions constitutin...
Text Solution
|
- In an ionic solid, B^(x-) ions constitute a ccp lattice, if A^(y+) ion...
Text Solution
|
- A binary solid A^(+) B^(-) has a structure with B^(-) ions constitutin...
Text Solution
|
- A binary solid A^(+)B^(-) has a structure with B^(-) ions constituting...
Text Solution
|