A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
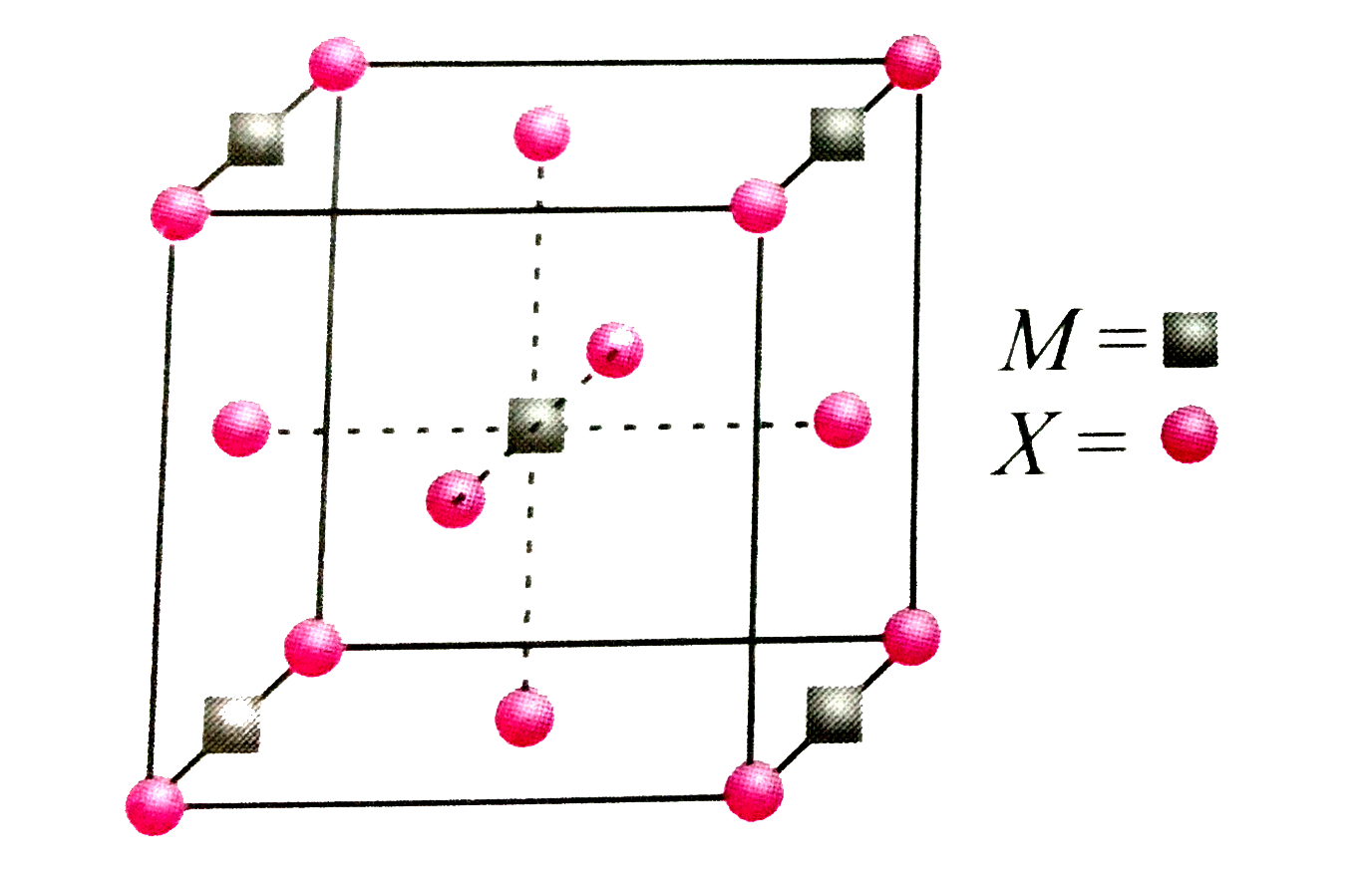
- A compound MpXq has cubic close packing (ccp) arrangement of X. Its un...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- A compound Mp Xq has cubic close packing (ccp) arrangement of X. Its u...
Text Solution
|
- एक यौगिक M(p) X(q) में X की घनीय संवृत संकुलन (ccp) व्यवस्था है । इसकी...
Text Solution
|
- What is the unit cell in a cubic close-packed (ccp) structure of parti...
Text Solution
|
- यौगिक M(p) X(q) में X के संदर्भ में घनीय निबिड़ संकुलित संरचना (ccp) की...
Text Solution
|
- यौगिक M(p)X(q), में X के संदर्भ में घनीय निबिड संकुलित संरचना (ccp) की...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|