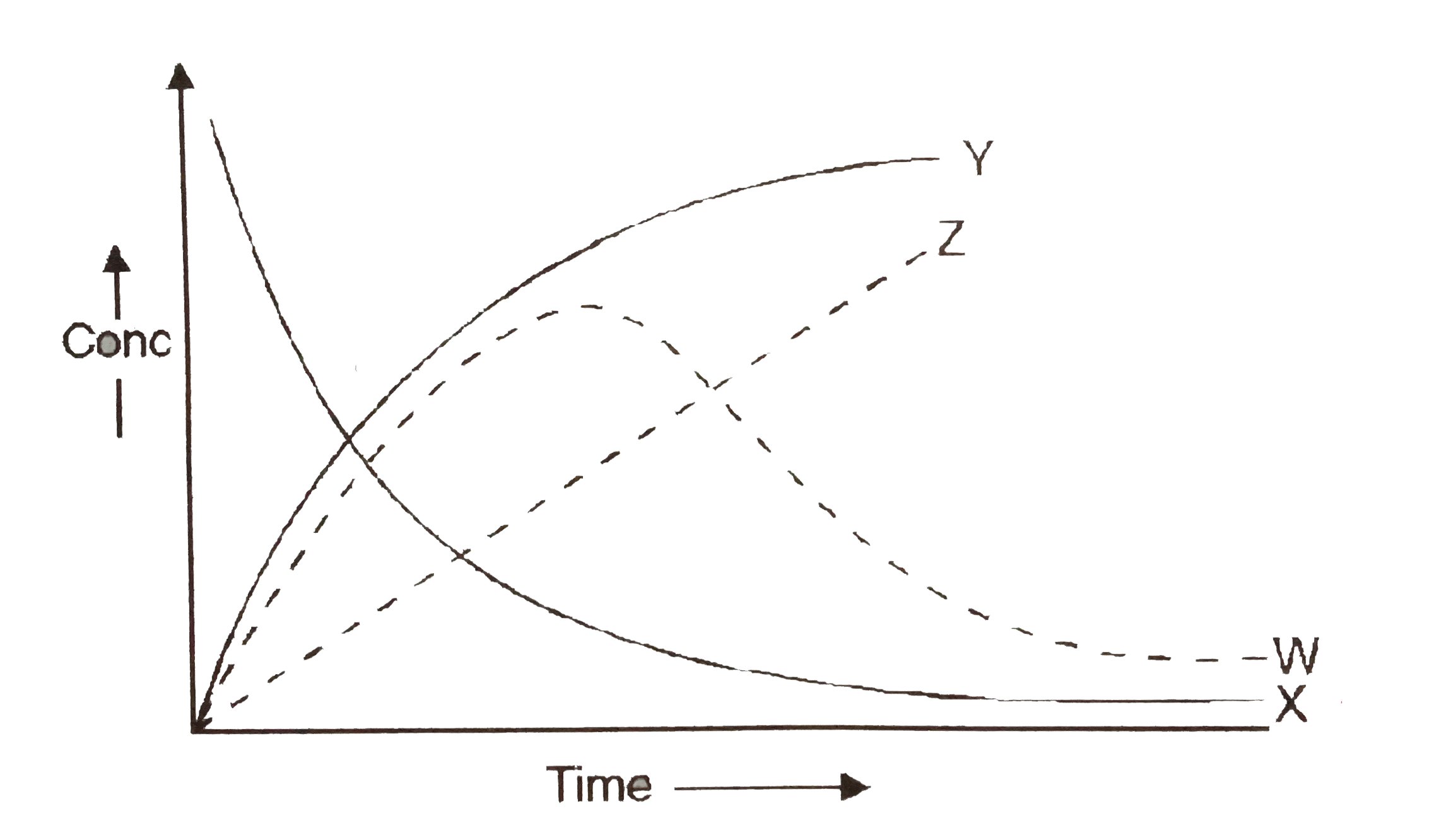
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction A+B rarr C+D The variation of the concentration of th...
Text Solution
|
- For the reaction, A+Brarr C+D . The variation of the concentration of ...
Text Solution
|
- For the reaction: A + B + Q hArr C + D, if the temperature is increa...
Text Solution
|
- For the reaction A+B rarr C+D The variation of the concentration of th...
Text Solution
|
- Given the following reaction involving A,B,C and D (i) C+B^(+)...
Text Solution
|
- At the point of intersection of the two curves shown for the reaction ...
Text Solution
|
- For the reaction, A + B to C + D. The variation of the concentration o...
Text Solution
|
- अभिक्रिया A + B+Q <--> C+D, के लिए, ताप बढ़ाने पर उत्पादों का सान्द्रण...
Text Solution
|
- For the reaction A+BtoC+D. The variation of the concentration of the p...
Text Solution
|