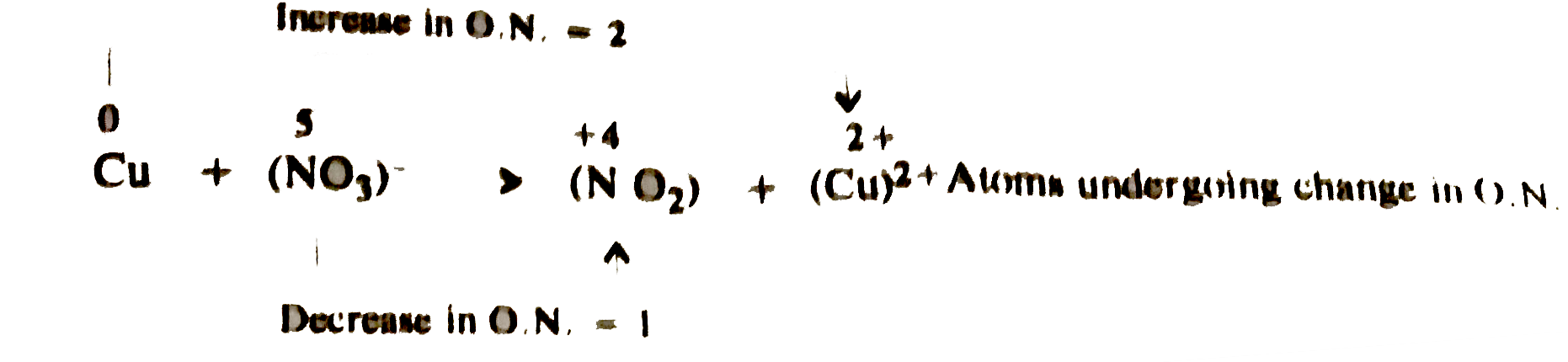Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Balance the following reaction in acidic medium. CuS+NO(3)^(ө)rarrcu...
Text Solution
|
- Balance the following reaction in acidic medium. CuS+NO(3)^(ө)rarrcu^(...
Text Solution
|
- Balance the following by ion electron method is basic medium. NO(3)^(ө...
Text Solution
|
- Balance the following by ion electron method (acidic medium). Mn^(2+)+...
Text Solution
|
- Balance the following by ion electron method in acidic medium. CIO(3)^...
Text Solution
|
- Balance the following reaction by ion electrons method ( acidic medium...
Text Solution
|
- Balance the following half-reactions in acidic medium: (a) IO(3)^(Ө)(a...
Text Solution
|
- Balance the following redox reaction : Cu+NO(3)^(-)rarrNO(2)+Cu^(2+) (...
Text Solution
|
- Balance the following chemical equation by partial equation method. Cu...
Text Solution
|