Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
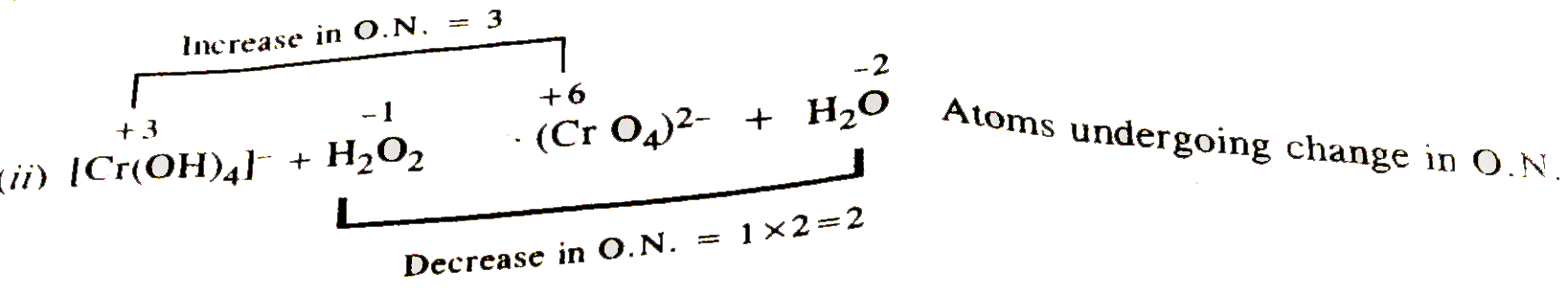
- Balance the following redox equaiton by both methods. [Cr(OH)(4)]^(ө...
Text Solution
|
- Balance the following by ion electron method (basic medium): Cr(OH)(...
Text Solution
|
- Balance the following equations: a. BaCrO(4) + KI+HClrarrBaCl(2)+I(2...
Text Solution
|
- Balance the following redox equaiton by both methods. [Cr(OH)(4)]^(ө)+...
Text Solution
|
- Balance the following chemical reactions (by ion electron method) (a...
Text Solution
|
- Balance the following redox reactions. Coppoer reacts with nitric ac...
Text Solution
|
- Balance the following equations by the ion electron method: a. MnO(4...
Text Solution
|
- Balance the following equation by oxidation number method PbS+H(2)O(2)...
Text Solution
|
- Balance the following redox equation: (i) Cu+NO(3)^(-)rarrrNO(2)+Cu^...
Text Solution
|
 Atoms undergoing change in O.N
Atoms undergoing change in O.N