A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION ENGLISH-PHOTOSYNTHESIS-CYG
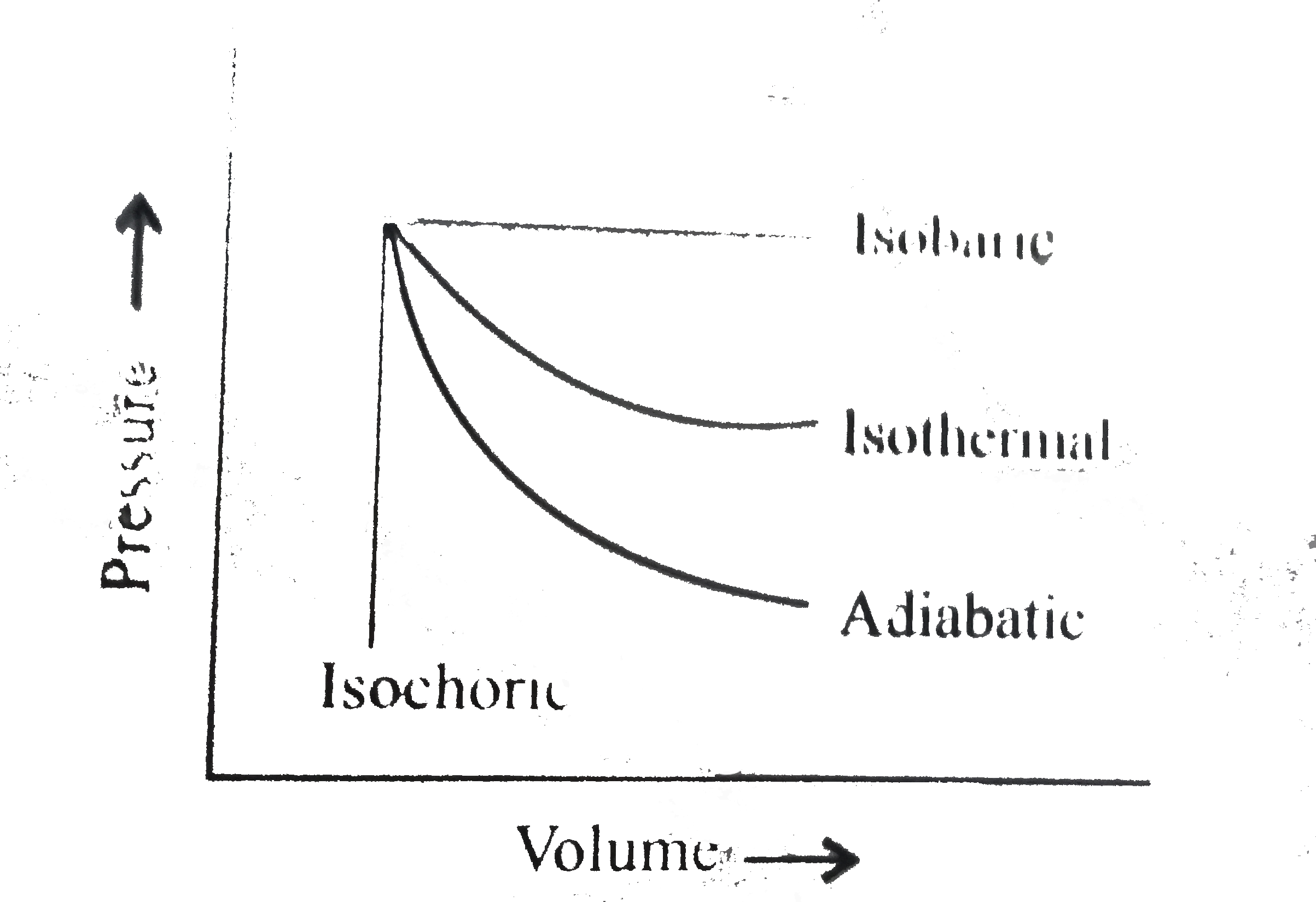
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- Solar radiations contain ultraviolet radiations of wavelength
Text Solution
|
- Soil humus theory of plant nourishment was given by
Text Solution
|
- Chlorophyll a is called universal photosynthetic pigment because it oc...
Text Solution
|
- Which one is Hill oxidant ?
Text Solution
|
- The action spectrum of photosynthesis was discovered by
Text Solution
|
- Girdling experiments cannot be successful in case of Cucurbita because...
Text Solution
|
- First transitory chemical formed by reaction between CO(2) and RuBP is
Text Solution
|
- RuBP occurs in
Text Solution
|
- The Scientist who discovered atmospheric CO(2) concentration to be sub...
Text Solution
|
- Wilmott's bubbler is meant for providing
Text Solution
|