Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-EXERCISE
- Chlorine has two isotopes, viz. Cl-35 and Cl-37 Their atomic masses ar...
Text Solution
|
- How is the problem regarding the position of cobalt("59CO) and nickel ...
Text Solution
|
- Answer the following questions: How did the position of get fixed t...
Text Solution
|
- Answer the following questions: Can there be an element with atomic ...
Text Solution
|
- Answer the following questions: What do you think? Should hydrogen be...
Text Solution
|
- Answer the following questions: The elements in the second period :Li...
Text Solution
|
- Answer the following questions: The elements in the third period , n...
Text Solution
|
- What is the relationship between the electronic configuration of an el...
Text Solution
|
- The atomic number of beryllium is 4 while that of oxygen is 8. Write d...
Text Solution
|
- What is the periodic trend in the variation of valency while going fro...
Text Solution
|
- Answer the following questions: Considering the elements of period 3...
Text Solution
|
- Answer the following questions: Considering the elements of period 3...
Text Solution
|
- Answer the following questions: Considering the elements of period 3 ...
Text Solution
|
- Answer the following questions: What is the periodic trend in the va...
Text Solution
|
- Classify the elements of the third period into metals and non-metals.
Text Solution
|
- Answer the following questions: On which side of the period are the m...
Text Solution
|
- On which side of the period did you find the Non-metals?
Text Solution
|
- What is the cause of non-metallic character of element?
Text Solution
|
- What is the expected trend in the variation of non-metallic character ...
Text Solution
|
- What would be the expected trend in the variation of non-metallic char...
Text Solution
|
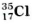 get fixed the modern periodic table?
get fixed the modern periodic table?