Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-EXERCISE
- Answer the following questions: Write down the electronic configurati...
Text Solution
|
- Answer the following questions: Write down the electronic configurati...
Text Solution
|
- Answer the following questions: The atomic number of aluminium is 13...
Text Solution
|
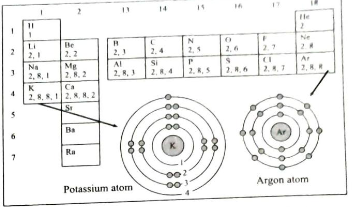
- Answer the following questions: Observe the following diagram and ans...
Text Solution
|
- Answer the following questions: Observe the following diagram and ans...
Text Solution
|
- Answer the following questions: Observe the following diagram and wri...
Text Solution
|
- Answer the following questions: Observe the following diagram and wri...
Text Solution
|
- Answer the following questions: Observe the following diagram and wri...
Text Solution
|
- Answer the following questions: Observe the following diagram and wri...
Text Solution
|
- Answer the following questions: Observe the following diagram and wri...
Text Solution
|
- Atomic radius goes on decreasing while going from left to right in a p...
Text Solution
|
- Give scientific reasons : Metallic character goes on decreassing wh...
Text Solution
|
- Give scientific reasons : Atomic radius goes on increasing down a g...
Text Solution
|
- Give scientific reasons : Elements belonging to the same group have...
Text Solution
|
- Give scientific reasons : Inert gases (zero group elements ) are ca...
Text Solution
|
- Give scientific reasons While going down the second group , the reac...
Text Solution
|
- Give scientific reasons : The third period contains only eight elem...
Text Solution
|
- Give scientific reasons : Fluroine is the most reactive in Group 17...
Text Solution
|
- Sodium is more metallic than Aluminium .
Text Solution
|
- Distinguish between: Mendeleev's periodic table and Modern periodic ...
Text Solution
|