Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION-METALLURGY-EXERCISE
- Answer the following questions: Extraction of mercury from its ore ci...
Text Solution
|
- Answer the following questions: Show the steps involved in the extrac...
Text Solution
|
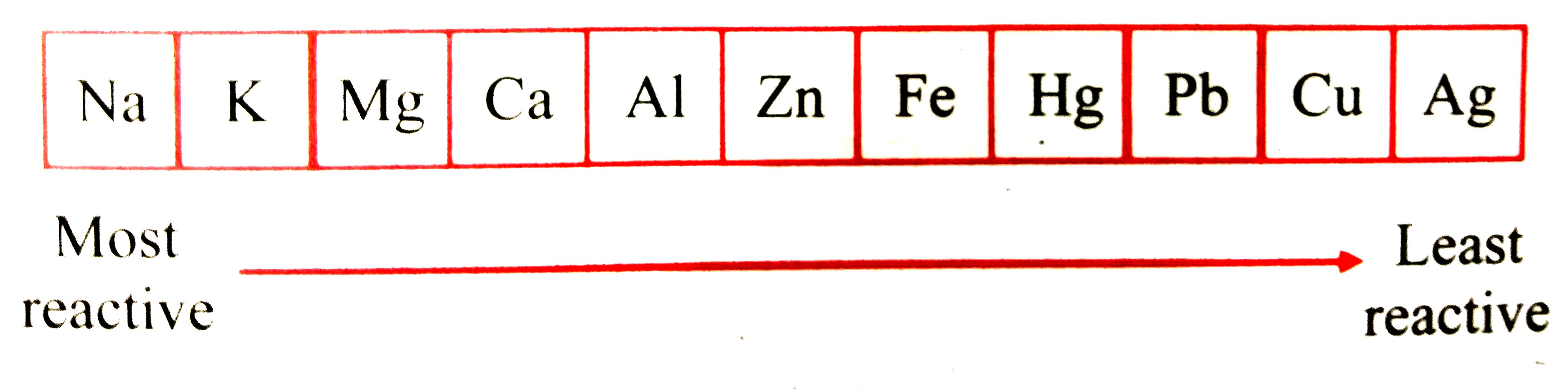
- In the given reactivity series, some metals are misplaced. Rearrange t...
Text Solution
|
- Answer the following questions: Explain the term corrosion with a sui...
Text Solution
|
- Answer the following questions: What is corrosion?
Text Solution
|
- Answer the following questions: Explain the different methods to prev...
Text Solution
|
- Answer the following questions: Write three methods of preventing rus...
Text Solution
|
- Explain the following: Why do silver articles turn balckish while c...
Text Solution
|
- Answer the following questions: Why do pure gold and platinum always...
Text Solution
|
- Answer the following in one or two sentences Which measures would y...
Text Solution
|
- Answer the following questions: What is done so to prevent rusting of...
Text Solution
|
- Explain the following: Can we permanently prevent the rusting of an...
Text Solution
|
- Answer the following questions: Why do new iron sheets appear shiny?
Text Solution
|
- Answer the following questions: What is meant by an alloy? Give two e...
Text Solution
|
- Answer the following questions: Write short notes on the following: G...
Text Solution
|
- Answer the following questions: Write short notes on the following: T...
Text Solution
|
- Answer the following questions: Write short notes on the following: E...
Text Solution
|
- Answer the following questions: Write short notes on the following: A...
Text Solution
|
- Answer the following questions: Write short notes on the following: A...
Text Solution
|
- Answer the following questions: In two methods of control of corrosio...
Text Solution
|