Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|49 VideosMETALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-18|1 VideosHYDROGEN
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|28 VideosSYNTHETIC FIBRES AND PLASTICS
S CHAND IIT JEE FOUNDATION|Exercise Self Assessment Sheet - 14|29 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-METALS AND NON METALS-QUESTION BANK-15
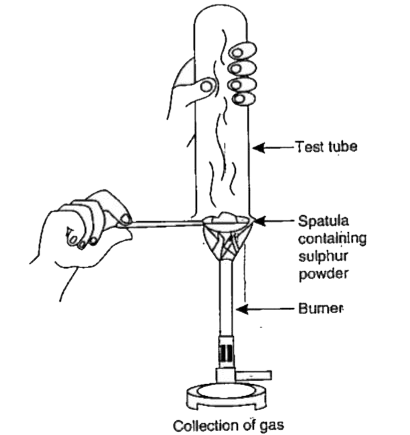
- Sameer took sulphur powder on a spatula and heated it. He collected th...
Text Solution
|
- Sameer took sulphur powder on a spatula and heated it. He collected th...
Text Solution
|
- Sameer took sulphur powder on a spatula and heated it. He collected th...
Text Solution
|
- You must have seen tarnished copper vessels being cleaned with lemon o...
Text Solution
|
- Why does magnesium ribbon start floating in hot water?
Text Solution
|
- Why do gallium and cesium melt in our palm?
Text Solution
|
- A metal with shining appearance reacts with hot water and also with di...
Text Solution
|
- From the set of metals- zinc, iron, copper and silver, select the fol...
Text Solution
|
- From among the set of metals-sodium, zinc, iron, copper and silver, se...
Text Solution
|
- From among the set of metals-sodium, zinc, iron, copper and silver, se...
Text Solution
|
- From among the set of metals-sodium, zinc, iron, copper and silver, se...
Text Solution
|
- Name one metal in following fitting the given description. Also, write...
Text Solution
|
- Name one metal in following fitting the given description. Also, write...
Text Solution
|
- Name one metal in following fitting the given description. Also, write...
Text Solution
|
- Name one metal in following fitting the given description. Also, write...
Text Solution
|
- Name one metal in following fitting the given description. Also, write...
Text Solution
|
- Zinc is extracted from zinc blende. The zinc blende is roasted. The so...
Text Solution
|
- Zinc is extracted from zinc blende. The zinc blende is roasted. The so...
Text Solution
|
- Zinc is extracted from zinc blende. The zinc blende is roasted. The so...
Text Solution
|
- Zinc is extracted from zinc blende. The zinc blende is roasted. The so...
Text Solution
|