Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|49 VideosMETALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-18|1 VideosHYDROGEN
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|28 VideosSYNTHETIC FIBRES AND PLASTICS
S CHAND IIT JEE FOUNDATION|Exercise Self Assessment Sheet - 14|29 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-METALS AND NON METALS-QUESTION BANK-15
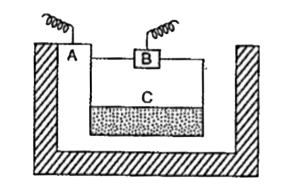
- The diagram shows the sketch of an electrolytic cell used in the extra...
Text Solution
|
- The diagram shows the sketch of an electrolytic cell used in the extra...
Text Solution
|
- The diagram shows the sketch of an electrolytic cell used in the extra...
Text Solution
|
- In the electrolytic refining of a metal M, what would you take as the ...
Text Solution
|
- On placing a piece of zinc metal in a solution of mercuric chloride, i...
Text Solution
|
- Name two metals that do not corroide easily. Give an example in suppor...
Text Solution
|
- Food cans are coated with tin and not with zinc because
Text Solution
|
- Four students A, B, C, and D took aqueous solutions of zinc sulphate a...
Text Solution
|
- Which of the following pairs will give displacement reactions?
Text Solution
|
- Which of the following methods is suitable for preventing an iron fryi...
Text Solution
|
- An element reacts with oxygen to give a compound with a high melting p...
Text Solution
|
- A piece of granulated zinc was dropped into copper sulphate solution. ...
Text Solution
|
- Give reason: Metals like palladium, silver and gold are used to make...
Text Solution
|
- Give reason: Sodium, potassium and lithium are stored under oil
Text Solution
|
- Give reason: Aluminium is a highly reactive metal, yet it is used to...
Text Solution
|
- give reason for the following: Carbonate and sulphide ores are usual...
Text Solution
|
- Give reason: Metals are regarded as electropositive elements
Text Solution
|
- Give reason: Carbon is not used for making aluminium from aluminium ...
Text Solution
|
- Give reason: Metals conduct electricity
Text Solution
|
- Metallic oxides of zinc, magnesium and copper were heated with the fol...
Text Solution
|