Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|49 VideosMETALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-18|1 VideosHYDROGEN
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|28 VideosSYNTHETIC FIBRES AND PLASTICS
S CHAND IIT JEE FOUNDATION|Exercise Self Assessment Sheet - 14|29 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-METALS AND NON METALS-QUESTION BANK-15
- Give reason: Carbon is not used for making aluminium from aluminium ...
Text Solution
|
- Give reason: Metals conduct electricity
Text Solution
|
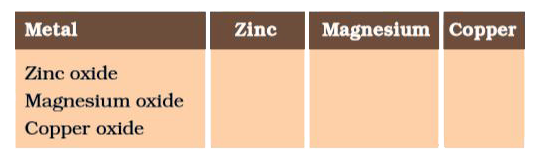
- Metallic oxides of zinc, magnesium and copper were heated with the fol...
Text Solution
|
- A student has been collecting silver coins and copper coins. One day s...
Text Solution
|
- A metal 'M' has the electronic configuration is 2, 8, 3 - and occurs i...
Text Solution
|
- Rusting of iron involves
Text Solution
|
- Which of the following metals can displace hydro-gen from dilute acids...
Text Solution
|
- The ore of which of the following metals can be concentrated by hydrau...
Text Solution
|
- Which of the following alloys is used for making magnets?
Text Solution
|
- Match the following:
Text Solution
|
- Which of the following metals was first discovered by man?
Text Solution
|
- When an iron nail gets rusted, iron oxide is formed
Text Solution
|
- Of the following metals which one pollutes the air of a big city?
Text Solution
|
- Which of the following is an incorrect statement?
Text Solution
|
- Which of the following is not a correct statement?
Text Solution
|
- Rust is
Text Solution
|
- The metal that is present of photo films is
Text Solution
|
- Amalgams are
Text Solution
|
- Identify the correct order of reactivity of metals among the following...
Text Solution
|
- Brass gets discoloured in air because of the presence of which of the ...
Text Solution
|