A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|49 VideosMETALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-18|1 VideosHYDROGEN
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|28 VideosSYNTHETIC FIBRES AND PLASTICS
S CHAND IIT JEE FOUNDATION|Exercise Self Assessment Sheet - 14|29 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-METALS AND NON METALS-QUESTION BANK-15
- Identify the incorrect statement about the alloys among the following:
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
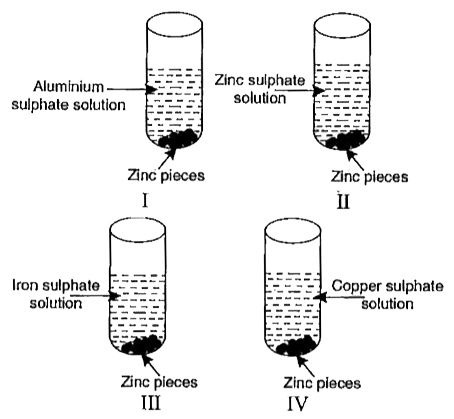
- Zinc pieces were placed in each of the four test tubes containing diff...
Text Solution
|
- Four metals Zn, Fe, Cu and Al are taken and added to the following sol...
Text Solution
|
- Draw a concept chart showing the processes involved during extraction ...
Text Solution
|
- Write balanced chemical equations for the following reaction Reducti...
Text Solution
|
- Write balanced chemical equations for the following reaction Calcium...
Text Solution
|
- Write balanced chemical equations for the following reaction Reactio...
Text Solution
|
- Write balanced chemical equations for the following reaction Reactio...
Text Solution
|
- Write balanced chemical equations for the following reaction Reactio...
Text Solution
|
- Write balanced chemical equations for the following reaction Reactio...
Text Solution
|
- Write balanced chemical equations for the following reaction Reducti...
Text Solution
|
- Write balanced chemical equations for the following reaction Reduct...
Text Solution
|
- Write balanced chemical equations for the following reaction Iron an...
Text Solution
|
- Write balanced chemical equations for the following reaction Zinc i...
Text Solution
|
- Write balanced chemical equations for the following reaction Dry ch...
Text Solution
|
- Write balanced chemical equations for the following reaction Burning...
Text Solution
|
- Write balanced chemical equations for the following reaction Zinc ca...
Text Solution
|
- Write balanced chemical equations for the following reaction Cinnaba...
Text Solution
|
- An ore on treatment with dilute hydrochloric acid gives a smell like t...
Text Solution
|