Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK (true or false)|18 VideosHYDROGEN
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK (Fill in the blanks by choosing the correct answer from the given alternatives)|9 VideosHYDROGEN
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|28 VideosCOMBUSTION AND FLAME
S CHAND IIT JEE FOUNDATION|Exercise Self Assessment Sheet-17(Fill in the blanks)|22 VideosMETALS AND NON METALS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET|49 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-HYDROGEN-QUESTION BANK
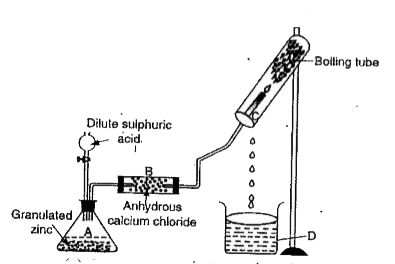
- The following apparatus is set up to show that hydrogen burns in air t...
Text Solution
|
- The following apparatus is set up to show that hydrogen burns in air t...
Text Solution
|
- The following apparatus is set up to show that hydrogen burns in air t...
Text Solution
|
- The following apparatus is set up to show that hydrogen burns in air t...
Text Solution
|
- Metallic hydrides react with water to form liquid 'A' and gas 'B'. In ...
Text Solution
|
- Metallic hydrides react with water to form liquid 'A' and gas 'B'. In ...
Text Solution
|
- Metallic hydrides react with water to form liquid 'A' and gas 'B'. In ...
Text Solution
|
- Match the statements in column A with those in column B.
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. I am a met...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. I am a meta...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. I am formed...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. I am a gas ...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. We are form...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. I am a met...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. It was I wh...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. Hydrogen pr...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. I am a flam...
Text Solution
|
- Fill in the boxes with alphabets to give correct answer. I am at th...
Text Solution
|
- Complete and balance the following equation : H2+"" rarr HCl
Text Solution
|
- Complete and balance the following equation : H2+S rarr
Text Solution
|