Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
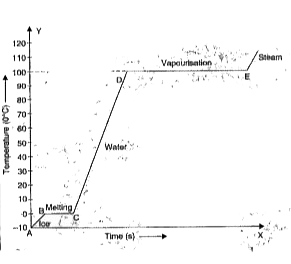
- A certain quantity of ice at 0^(@)C is heated till it changes into ste...
Text Solution
|
- A block of ice at -10^@C is slowly heated and converted to steam at 10...
Text Solution
|
- The following graph represents change of state of 1 gram of ice at -20...
Text Solution
|
- 0^(@)C ताप कि बर्फ को गर्म करके 100^(@)C ताप की भाप में परिवर्तित किया...
Text Solution
|
- .0^@C ताप की बर्फ को गर्म करके 100^@C ताप को भाप में परिवर्तित किया जा...
Text Solution
|
- 0^@C की बर्फ के निश्चित द्रव्यमान को एक नियत सामर्थ्य वाले हीटर से धी...
Text Solution
|
- A block of ice at -5^@C is slowly heated and converted to steam at 10...
Text Solution
|
- A block of ice -10^(@)C is slowly heated and converted to steam at 100...
Text Solution
|
- बर्फ के गलन की गुप्त ऊष्मा 80 कैलोरी/ग्राम तथा भाप की गुप्त ऊष्मा 540 ...
Text Solution
|