Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
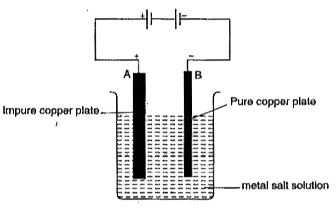
- The diagram shows the process of purification of copper metal. A thick...
Text Solution
|
- Fill in the blanks. . If you pass current through copper sulphate solu...
Text Solution
|
- The process that you see is used for purification of copper. A thin pl...
Text Solution
|
- ਜੇ ਕਾੱਪਰ ਸਲਫੇਟ ਦੇ ਘੋਲ਼ ਵਿੱਚੋਂ ਬਿਜਲੀ ਧਾਰਾ ਲੰਘਾਈ ਜਾਵੇ ਤਾਂ ਕਾੱਪਰ ਬੈਟਰੀ ਦੇ ...
Text Solution
|
- Fill in the blanks. . If you pass current through copper sulphate solu...
Text Solution
|
- The process that you saw in activity 14.7 is used for purification of ...
Text Solution
|
- Fill in the blanks : If you pass current through copper sulphate sol...
Text Solution
|
- If you pass current through copper sulphate solution, copper deposited...
Text Solution
|
- Copper metal is not used:
Text Solution
|