A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CEL: THE BASIC UNIT OF LIFE
CENGAGE BIOLOGY|Exercise CHALLENGING EXERCISE|4 VideosCEL: THE BASIC UNIT OF LIFE
CENGAGE BIOLOGY|Exercise CONSOLIDATED EXERCISE (Multiple Choice Questions with More Than One Correct Answer)|64 VideosBIOLOGICAL CLASSIFICATION
CENGAGE BIOLOGY|Exercise CHALLENGING EXERCISE|1 VideosCELL CYCLE AND CELL DIVISION
CENGAGE BIOLOGY|Exercise CHALLENGING EXERCISE |6 Videos
Similar Questions
Explore conceptually related problems
CENGAGE BIOLOGY-CEL: THE BASIC UNIT OF LIFE-OLYMPIAD AND NTSE LEVEL EXERCISES
- Chromosome must condense to approximately 1/500th of their length for ...
Text Solution
|
- Assertion. The two chains of DNA molecule are antiparallel. Reason. ...
Text Solution
|
- Which of the following is correct for the given figure?
Text Solution
|
- Check each of the following statements that are true with regard to ch...
Text Solution
|
- Refer to the given keys and answer the following question. P. memb...
Text Solution
|
- There is a garden which requires water supply. Here is a pipe which is...
Text Solution
|
- Keeping in view the fluid mosaic model for the stucture of cell membra...
Text Solution
|
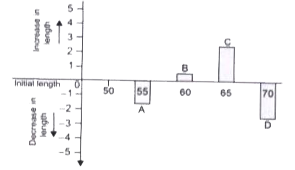
- A graph was plotted between the initial length of strips and the chang...
Text Solution
|
- In Column I, names of eminent scientists have been given while in Colu...
Text Solution
|