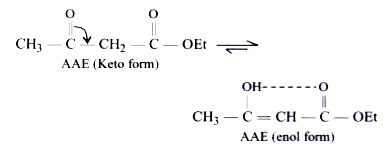
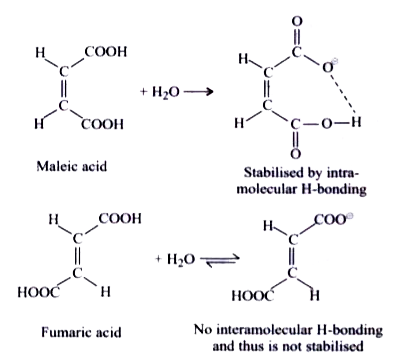
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
CENGAGE CHEMISTRY|Exercise OLYMPIAD AND NTSE LEVEL EXERCISES|9 VideosCHEMICAL BONDING
CENGAGE CHEMISTRY|Exercise CHALLENGING EXERCISE|6 VideosCHEMICAL ARITHMETIC
CENGAGE CHEMISTRY|Exercise MANDATORY EXERCISE (EXERCISE SET III) (OLYMPIAD AND NTSE LEVEL EXCERCISES)|10 VideosCHEMICAL REACTIONS
CENGAGE CHEMISTRY|Exercise OLYMPIAD AND NTSE LEVEL EXCERCISES|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING-OLYMPIAD AND NTSE LEVEL EXERCISES (Paragraph)
- Hydrogen bond is a weak bond formed between hydrogen atoms and highly ...
Text Solution
|
- Hydrogen bond is a weak bond formed between hydrogen atoms and highly ...
Text Solution
|
- Hydrogen bond is a weak bond formed between hydrogen atoms and highly ...
Text Solution
|
- Hydrogen bond is a weak bond formed between hydrogen atoms and highly ...
Text Solution
|