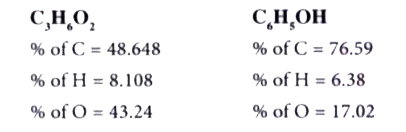Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL ARITHMETIC
CENGAGE CHEMISTRY|Exercise MANDATORY EXERCISE (EXERCISE SET III) (OLYMPIAD AND NTSE LEVEL EXCERCISES)|10 VideosCHEMICAL ARITHMETIC
CENGAGE CHEMISTRY|Exercise MANDATORY EXERCISE (EXERCISE SET II) |31 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise OLYMPIAD AND NTSE LEVEL EXERCISES|10 VideosCHEMICAL BONDING
CENGAGE CHEMISTRY|Exercise OLYMPIAD AND NTSE LEVEL EXERCISES|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL ARITHMETIC -MANDATORY EXERCISE (EXERCISE SET III)
- Calculate the relative molecular mass of the substance: Cl (2)
Text Solution
|
- Calculate the percentage composition by mass of propanoic acid, C (3) ...
Text Solution
|
- Calculate the molecular/formula mass of each of the substances, given ...
Text Solution
|
- Calculate the mass percent composition of each of the substance: Sul...
Text Solution
|
- Calculate the mass percent composition of each of the substance: Pot...
Text Solution
|
- Calculate the mass percent composition of each of the substance: Glu...
Text Solution
|
- Calculate the mass percent composition of each of the substance: Ure...
Text Solution
|
- If a sample of copper contains 69.1% of 63 Cu of isotopic mass 62.930...
Text Solution
|
- The percentage of water of crystallisation in washing soda Na (2) CO(3...
Text Solution
|
- What is the mass percent of oxygen in Al (2) (SO(4)) (3). 18 H(2) O ? ...
Text Solution
|
- The oxidation state of Xe in Xe OF(2), XeF(4) and Xe O (2) is
Text Solution
|
- What is the relation between atomic mass and number of nucleon ?
Text Solution
|
- Write the atomic mass, atomic weight and relative atomic mass of calci...
Text Solution
|
- Calculate the no. of atoms of He in 56 amu of He simple.
Text Solution
|
- What is meant by 1 g atom of Na ?
Text Solution
|
- Express the relation between atomic mass and gram atomic weight ?
Text Solution
|
- Calculate the % mass of C in H(2) C (2) O(4) 2H(2)O
Text Solution
|
- Calculate of % mass of H(2) O in Mg SO(4) .7H(2)O sample.
Text Solution
|
- Calcualte the weight of H(2)O present in 100 g sample of Mg SO(4). 7H(...
Text Solution
|
- If two compounds have same Empirical form then mass percentage of both...
Text Solution
|