Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REACTION KINETICS AND CHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise OLYMPIAD AND NTSE LEVEL EXERCISES|10 VideosREACTION KINETICS AND CHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise MULTIPLE CHOICE QUESTIONS WITH ONE OR MORE THAN ONE CORRECT ANSWER|5 VideosRADIOACTIVITY
CENGAGE CHEMISTRY|Exercise OLYMPIAD AND NTSE LEVEL EXERCISES|10 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
CENGAGE CHEMISTRY|Exercise OLYMPIAD AND NTSE LEVEL EXERCISES |10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REACTION KINETICS AND CHEMICAL EQUILIBRIUM-CHALLENGING EXERCISE
- One mol each of A and B are heated in a 2-dm^(3) container. At equilib...
Text Solution
|
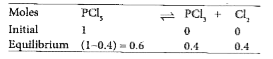
- One mol of PCl(5) is heated in a 2-dm^(3) container. At equilibrium, 4...
Text Solution
|
- 2A+B+CtoA(2)B+C rate = k[A][B] with k=2.0xx10^(-6)mol^(-2)s^(-1) (...
Text Solution
|
- 2 mol of hydrogen iodide are heated in a 2-dm^(3) container. At equili...
Text Solution
|
- 0.087 mol of NO and 0.0437 mol of Br(2) are mixed in a closed containe...
Text Solution
|