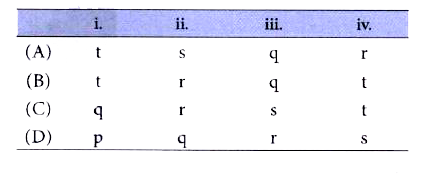
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
CENGAGE PHYSICS-HEAT AND TEMPERATURE -OLYMPIAD AND NTSE LEVEL EXERCISES
- A pendulum clock keeps correct time at 0^(@)C. Its mean coefficient of...
Text Solution
|
- A vertical column 50 cm long at 50^(@)C balances another column of sam...
Text Solution
|
- Two liquid A and B are at 32^@C and 24^@C. When mixed in equal masses ...
Text Solution
|
- 0.93 watt - hour of energy is supplied to a block of ice weighing 10 g...
Text Solution
|
- 300 grams of water at 25^@ C is added to 100 grams of ice at 0^@ C. Th...
Text Solution
|
- An iron tyre is to be fitted onto a wooden wheel 1.0 m in diameter. Th...
Text Solution
|
- Two rods having length l(1) and l(2) made of materials with the linea...
Text Solution
|
- In a container of negligible heat capacity, 200 g ice at 0^(@)C and 10...
Text Solution
|
- In a container of negligible heat capacity, 200 g ice at 0^(@)C and 10...
Text Solution
|
- In a container of negligible heat capacity, 200 g ice at 0^(@)C and 10...
Text Solution
|