A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-QUESTION PAPER 2013-PART-II ( CHEMISTRY)
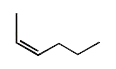
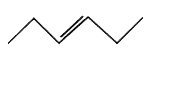
- The structure of cis-3-hexene is
Text Solution
|
- The major product obtained in the reaction of aniline with acetic anhy...
Text Solution
|
- The maximum number of isomers that can result from monobromination of ...
Text Solution
|
- The compound X(C(7)H(9)N) reacts with benzensulfonyl chloride to give ...
Text Solution
|
- In 108 g of water, 18 g of a non-volatile compound is dissolved. At 10...
Text Solution
|
- The standard electrode potential of Zn^(2+) // Zn is -0.76V and that...
Text Solution
|
- Consider the equilibria (1) and (2) with equilibrium constants K1 and ...
Text Solution
|
- Aqueous solution of metallic nitrate X reacts with NH4OH to form Y whi...
Text Solution
|
- The density of eq. wt of a metal are 10.5 g cm^(-3) and 100, respectiv...
Text Solution
|
- The amount of Na2S2O3. 5H2O required to completely reduce 100 mL of 0...
Text Solution
|
- In aqueous solution, [Co(H2O)6]^(2+) (X) reacts with molecular oxygen ...
Text Solution
|