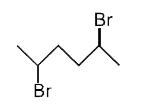
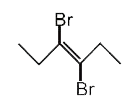
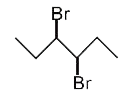
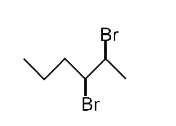
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
QUESTION PAPER 2013
KVPY PREVIOUS YEAR|Exercise PART-I ( CHEMISTRY)|20 VideosQUESTION PAPER 2013
KVPY PREVIOUS YEAR|Exercise PART-II ( CHEMISTRY)|10 VideosQUESTION PAPER 2013
KVPY PREVIOUS YEAR|Exercise PART-II ( CHEMISTRY)|10 VideosMOCK TEST 9
KVPY PREVIOUS YEAR|Exercise EXERCISE|26 VideosQUESTION PAPER 2020
KVPY PREVIOUS YEAR|Exercise PART-II(CHEMISTRY)|10 Videos
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-QUESTION PAPER 2013-PART-II (CHEMISTRY)
- The degree of dissociation of acetic acid (0.1 mol L^(-1)) in water (K...
Text Solution
|
- Compound 'X' on heating with Zn dust gives compound 'Y' which on trea...
Text Solution
|
- The amount of metallic Zn (Atomic weight = 65.4) required to react wit...
Text Solution
|
- Natural abundances of ""^(12)C and ""^(13)C isotopes of carbon are 99%...
Text Solution
|
- 2, 3-Dimethylbut-2-ene when reacted with bromine forms a compound whic...
Text Solution
|