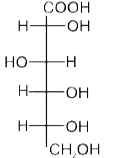
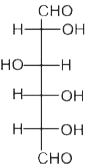
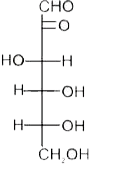
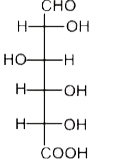
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-QUESTION PAPER 2013-PART-I ( CHEMISTRY)
- Among the following, the set of isoelectronic ions is
Text Solution
|
- For a zero-order reaction with rate constant k, the slope of the plot ...
Text Solution
|
- The compound which reacts with excess bromine to produce 2, 4, 6-tribr...
Text Solution
|
- Ethyl acetate reacts with NH2NHCONH2 to form
Text Solution
|
- The variation of solubility of four different gases (G1, G2, etc.) in ...
Text Solution
|
- For the reaction, A iff n B the concentration of A decreases from 0.0...
Text Solution
|
- The reaction of ethyl methyl ketone with Cl(2)//"excess" OH^(-) gives ...
Text Solution
|
- The compound that readily tautomerizes is
Text Solution
|
- Hydrolysis of BCl3 gives X which on treatment with sodium carbonate pr...
Text Solution
|
- The numbers of lone pair(s) on Xe in XeF2 and XeF4 are, respectively
Text Solution
|
- The entropy change in the isothermal reversible expansion of 2 moles o...
Text Solution
|
- D-Glucose upon treatment with bromine-water gives
Text Solution
|
- In the structure of borax, the numbers of boron atoms and B-O-B units,...
Text Solution
|
- The number of peptide bonds in the compound is
Text Solution
|
- For the isothermal reversible expansion of an ideal gas
Text Solution
|
- If the angle of incidence of X-ray of wavelength 3Å which produces a s...
Text Solution
|
- The number of ions produced in water by dissolution of the complex hav...
Text Solution
|
- The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-)...
Text Solution
|
- The order of ,SN 1 reactivity in aqueous acetic acid solution for the ...
Text Solution
|
- An ionic compound is formed between a metal M and a non-metal Y. If M ...
Text Solution
|