Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JBD PUBLICATION-Thermodynamics-EXAMPLE
- A tyre is pumped to a pressure of 3.375 atmospheres and at 27^@C sudde...
Text Solution
|
- Assuming a domestic refrigerator a reversible engine working between m...
Text Solution
|
- Give two statements of second law of thermodnamics.
Text Solution
|
- Write short note on cyclic process.
Text Solution
|
- 5 moles of oxygen are heated at constant volume from 10^@C ot 20^@C.Wh...
Text Solution
|
- The molar heat capacity of a gas at constant volume is to be 5 cal mol...
Text Solution
|
- 0.32 g of oxygen is kept in a rigid container and is heated .Find the ...
Text Solution
|
- What do you understand by isobaric and isochoric process?
Text Solution
|
- What is the relation between CP and Cv?
Text Solution
|
- How can you explain that Cp is greatere than Cv ?
Text Solution
|
- Write detail of specific heats of gases.
Text Solution
|
- A gas expands in such a manner that its pressure and volume comply wit...
Text Solution
|
- A gas occupying one litre at 80 cm pressure is expanded adiabatically ...
Text Solution
|
- If at 50^@C and 75 cm of mercury pressure , a definite mass of a gas ...
Text Solution
|
- Write short note on thermal equilibrium.
Text Solution
|
- Consider a PV-diagram in which the path followed by one mole of perfec...
Text Solution
|
- Consider a PV-diagram in which the path followed by one mole of perfec...
Text Solution
|
- Consider a PV-diagram in which the path followed by one mole of perfec...
Text Solution
|
- The initial state of a certain gas is (Pi,Vi,Ti). It undergoes expansi...
Text Solution
|
- The initial state of a certain gas is (Pi,Vi,Ti). It undergoes expansi...
Text Solution
|
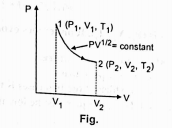 Given to internal enregy for one mole of gas at temperature T is `(3 // 2)` RT,find the heat supplied to the gas when it is taken from state 1 to 2,with `V_2=2V_2`.
Given to internal enregy for one mole of gas at temperature T is `(3 // 2)` RT,find the heat supplied to the gas when it is taken from state 1 to 2,with `V_2=2V_2`.