Similar Questions
Explore conceptually related problems
JBD PUBLICATION-Behaviour of Perfect Gases and Kinetic Theory of Gases-EXERCISE
- Two different gases have exactly the same temperature. Does this mean ...
Text Solution
|
- What is an ideal gas ?
Text Solution
|
- The Sl unit of gas constant is:
Text Solution
|
- STATEMENT-1 : The average translational kinetic energy per molecule of...
Text Solution
|
- Air is trapped in a horizontal glass tube by 36 cm mercury column as s...
Text Solution
|
- What is the average kinetic energy. Of the molecule of any gas at 100^...
Text Solution
|
- Calculate the most probable speed of a molecule of hydrogen at N.T.P. ...
Text Solution
|
- The speed of 8 particles are 1, 1, 1.5, 1, 2, 2, 1, 3 cm s^-1. Calcula...
Text Solution
|
- The speed of 8 particles are 1, 1, 1.5, 1, 2, 2, 1, 3 cm s^-1. Calcula...
Text Solution
|
- The speed of 8 particles are 1, 1, 1.5, 1, 2, 2, 1, 3 cm s^-1. Calcula...
Text Solution
|
- Derive ideal gas equation.
Text Solution
|
- The volume of a gas sample is increased. Why does the pressure which i...
Text Solution
|
- In the light of kinetic theory of gases, why pressure of container inc...
Text Solution
|
- Explain the phenomenon of evaporation on the basis of kinetic theory o...
Text Solution
|
- Why there is practically no atmosphere near the surface of the moon?
Text Solution
|
- In the light of kinetic theory of gases, why pressure of container inc...
Text Solution
|
- Explain with the help of kinetic theory, why pressure of a gas in its ...
Text Solution
|
- In the derivation of perfect gas equation, the effect of gravity has n...
Text Solution
|
- Do diatomic gases have same specific heat at constant volume as monoat...
Text Solution
|
- A vessel contains two non-reactive gases, neon (monatomic) and oxygen ...
Text Solution
|
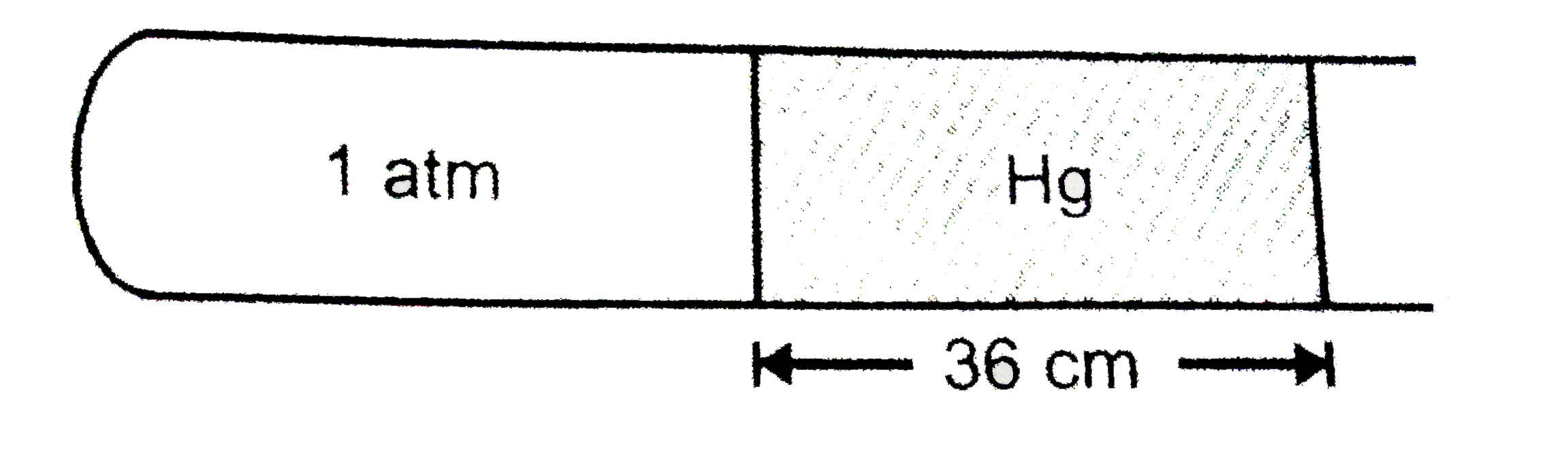 If the tube is held vertical keeping the open end up, lengh of air column shrink to 19 cm. What is the lengh (in cm) by which the mercury column shifts down?
If the tube is held vertical keeping the open end up, lengh of air column shrink to 19 cm. What is the lengh (in cm) by which the mercury column shifts down?