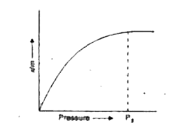
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-SURFACE CHEMISTRY -Question Bank
- Differentiate between: Adsorption Isotherm and Adsorption Isobar.
Text Solution
|
- What is adsorption isotherm ?
Text Solution
|
- Write Freundlich adsorption isotherm equation at high pressures.
Text Solution
|
- Write Freundlich adsorption isotherm equation at high pressures.
Text Solution
|
- What is adsorption isotherm ?
Text Solution
|
- Can adsorption occur from solutions ? Give examples.
Text Solution
|
- How does adsorption of gases on solids depend upon: Temperature?
Text Solution
|
- Explain two applications of adsorption.
Text Solution
|
- Define Catalyst and Catalysis.
Text Solution
|
- What is heterogeneous catalysis ? What role does adsorption play in he...
Text Solution
|
- Give the mechanism of heterogeneous catalysed reaction.
Text Solution
|
- Give four examples of heterogeneous catalysis.
Text Solution
|
- What name is given to the catalysis when the state of the catalyst is ...
Text Solution
|
- What name is given to the catalysis when the state of the catalyst is ...
Text Solution
|
- What name is given to the catalysis which is explained by lock-and-key...
Text Solution
|
- Write two differences between homogeneous catalysis and heterogeneous ...
Text Solution
|
- What is homogeneous and heterogeneous catalysis ? Give one example of ...
Text Solution
|
- Write two differences between homogeneous catalysis and heterogeneous ...
Text Solution
|
- Explain briefly homogeneous catalysis.
Text Solution
|
- What do you understand by activity and selectivity of a catalyst ? Giv...
Text Solution
|