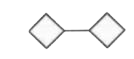
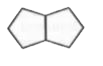
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the compounds shown below, is not an isomer of the others...
Text Solution
|
- Which compounds shown below has six isomers?
Text Solution
|
- From the compound shown below, choose which is/are aromatic.
Text Solution
|
- Which one of following pairs of compounds are functional isomers of ea...
Text Solution
|
- Which of the compound shown below are isomers?
Text Solution
|
- Which one of the following compounds give only one isomer upon nitrati...
Text Solution
|
- Which one of the following compounds give only one isomer upon nitrati...
Text Solution
|
- Which one of the compound is not isomers of others?
Text Solution
|
- Among the following compounds, which is not a structural isomers of ot...
Text Solution
|