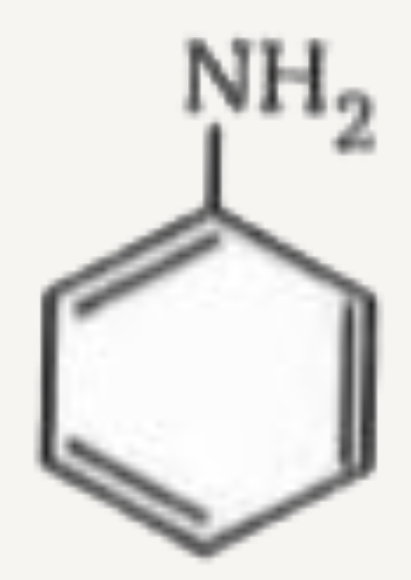
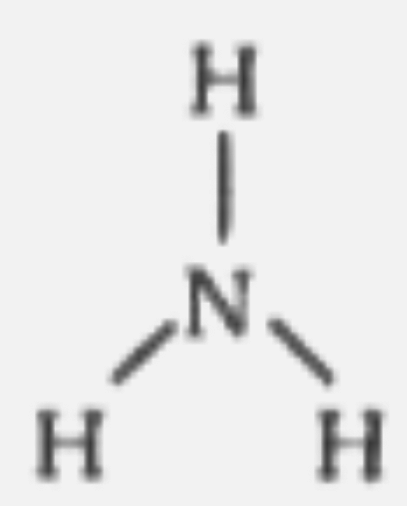
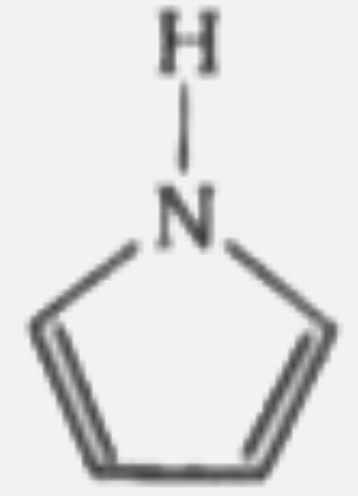
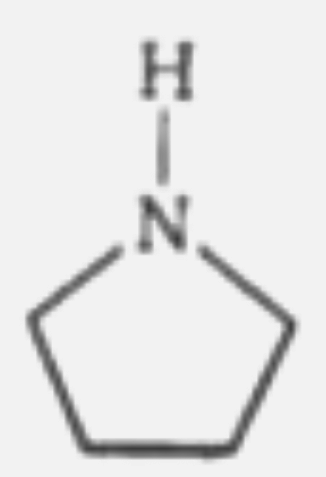
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is the strongest Bronsted base ?
Text Solution
|
- The strongest Bronsted base in the following anion is
Text Solution
|
- The strongest Bronsted base in the following anion is:
Text Solution
|
- Strongest Bronsted base is
Text Solution
|
- Among the following amines, the strongest Bronsted base is
Text Solution
|
- Which one of the following anionc is the strongest Bronsted base ?
Text Solution
|
- Which of the following is strongest Bronsted base
Text Solution
|
- Which one among the following in the strongest Bronsted base.
Text Solution
|
- निम्न में से कौन - सा ऋणायन प्रबलतम ब्रॉन्सटेड क्षार है ?
Text Solution
|