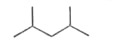
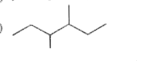
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following substances is not an isomer of 3-ethyl 2-methyl...
Text Solution
|
- Assertion (A): Pentane and 3 methyl pentane are chain isomers. Reason ...
Text Solution
|
- What is wrong with the following names ? Drawn the structures they rep...
Text Solution
|
- Which compound is not the isomer of 3-Ethyle-2- methylpentane?
Text Solution
|
- Which sequence of step describes the best synthesis of 2 - methyl-3- p...
Text Solution
|
- Which of the following is not a isomer of pentane ?
Text Solution
|
- 2-एथाक्सि-3 मिथाइल पेन्टेन की विलियमसन संश्लेषण क्रिया लिखिये | एथेनॉल...
Text Solution
|
- Which of the following is the structure of 3-ethyl pentane?
Text Solution
|
- which of the following is not an isomer of pentanal
Text Solution
|