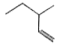
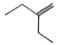
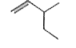
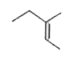
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following alkenes is the most stable ?
Text Solution
|
- Which of the following alkenes is most stable?
Text Solution
|
- Which of the following alkene is most stable alkene.
Text Solution
|
- Which of the following reaction produces most stable alkene ?
Text Solution
|
- Which among the following alkenes will be most stable :
Text Solution
|
- Which of the following is most stable alkene ?
Text Solution
|
- Which of the following is most stable alkene ?
Text Solution
|
- Which of the following is the most stable alkene
Text Solution
|
- Which of the following alkenes is most stable-
Text Solution
|