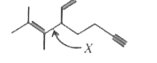
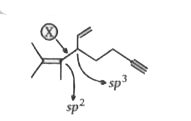
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Bond X is made by the overlap of which type of hybridized orbitals ?
Text Solution
|
- What orbitals can overlap to form a sigma -bond and which orbitals can...
Text Solution
|
- Which of the following has a bond formed by overlap of sp^(3)-sp, hybr...
Text Solution
|
- दो p- कक्षकों (ऑर्बिटलों ) के अक्षीय अतिव्यापन से किस प्रकार का आबन...
Text Solution
|
- Calculate value of |x-y|, here x and y are the total number of bonds i...
Text Solution
|
- Calculate value of |x-y| , here x and y are the total number of bonds ...
Text Solution
|
- Orbital Overlap Concept | Types Of Overlapping And Nature Of Covalent ...
Text Solution
|
- Cbse | Orbital Overlap Concept | Types Of Overlapping And Nature Of Co...
Text Solution
|
- Which of the following has a bond formed by the overlap of sp-sp^(3) h...
Text Solution
|