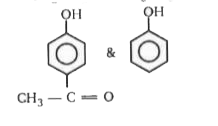
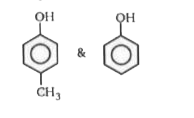
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Among the given pairs, in which pair second compound is more acidic th...
Text Solution
|
- Among the following pair of reactions in which pair second reaction is...
Text Solution
|
- Among the given pairs in which pairs first compound has higher boiling...
Text Solution
|
- Among the given pairs ,in which pair first compound reacts faster than...
Text Solution
|
- In the the given pair of compound , in which pair the second compound ...
Text Solution
|
- In which pairs first compound is stronger acid than the second ?
Text Solution
|
- In the given pair compounds in which pair second cpmpound has highter ...
Text Solution
|
- From among following pairs, number of pairs in which first compound is...
Text Solution
|
- किस युग्म में प्रथम यौगिक, द्वितीय की तुलना में अधिक प्रबल अम्ल है ?
Text Solution
|